��Ŀ����
�ҹ���ѧ���ڡ�����(�������������黯����)���³����������о���ȡ������Ҫ�ɹ������о���Ŀ�ٻ�2013��ȡ�������Ȼ��ѧ����һ�Ƚ���
��1����̬Fe2+�ĺ�������Ų�ʽΪ_________________��
��2����������������Ԫ���е縺��ֵ�ɴ�С��˳����__________(����Ӧ��Ԫ�ط������)��
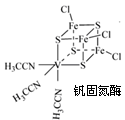
��3��Fe(SCN)3��Һ�м���NH4F���������·�Ӧ��Fe(SCN)3+6NH4F=(NH4)3FeF6+3NH4SCN��
��(NH4)3FeF6���ڵ����������������ۼ����_________(ѡ����ţ���ͬ)��
a����λ�� b����� c�������� d�����Ӽ�
����֪SCNһ�и�ԭ������������8�����ȶ��ṹ����Cԭ�ӵ��ӻ���ʽΪ_____________,��ԭ������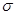 ����
����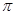 �����ı�ֵΪ___________________��
�����ı�ֵΪ___________________��
��4��FeCl3����������ˮ���Ҵ����þƾ��Ƽ��ȼ�����������FeF3�����۵����1000oC���Խ������ֻ������۵����ϴ��ԭ��_______________________________��
��5�������ס�����Ϊͬ����Ԫ�أ����仯����Ľṹ�������Ƕ������ġ�
�ٸ����⻯��RH3(NH3��PH3��AsH3)��ij��������R�ĺ˵�����ı仯��������ͼ��ʾ����Y��ɱ�ʾ���⻯��(RH3)���ʿ�����________��
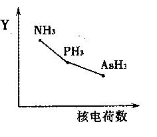
a���ȶ��� b���е� c��R��H���� d�����Ӽ�������
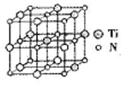
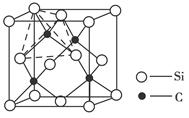
��̼�����ѻ���������������ͺ��պ���������й㷺��Ӧ�ã���ṹ����̼ԭ��ȡ�������Ѿ���(�ṹ��ͼ)����ĵ�ԭ�ӣ��ݴ˷���������̼�����ѻ�̨��Ļ�ѧʽΪ______��
��1����̬Fe2+�ĺ�������Ų�ʽΪ_________________��
��2����������������Ԫ���е縺��ֵ�ɴ�С��˳����__________(����Ӧ��Ԫ�ط������)��
��3��Fe(SCN)3��Һ�м���NH4F���������·�Ӧ��Fe(SCN)3+6NH4F=(NH4)3FeF6+3NH4SCN��
��(NH4)3FeF6���ڵ����������������ۼ����_________(ѡ����ţ���ͬ)��
a����λ�� b����� c�������� d�����Ӽ�
����֪SCNһ�и�ԭ������������8�����ȶ��ṹ����Cԭ�ӵ��ӻ���ʽΪ_____________,��ԭ������
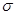 ����
����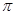 �����ı�ֵΪ___________________��
�����ı�ֵΪ___________________����4��FeCl3����������ˮ���Ҵ����þƾ��Ƽ��ȼ�����������FeF3�����۵����1000oC���Խ������ֻ������۵����ϴ��ԭ��_______________________________��
��5�������ס�����Ϊͬ����Ԫ�أ����仯����Ľṹ�������Ƕ������ġ�
�ٸ����⻯��RH3(NH3��PH3��AsH3)��ij��������R�ĺ˵�����ı仯��������ͼ��ʾ����Y��ɱ�ʾ���⻯��(RH3)���ʿ�����________��

a���ȶ��� b���е� c��R��H���� d�����Ӽ�������
��̼�����ѻ���������������ͺ��պ���������й㷺��Ӧ�ã���ṹ����̼ԭ��ȡ�������Ѿ���(�ṹ��ͼ)����ĵ�ԭ�ӣ��ݴ˷���������̼�����ѻ�̨��Ļ�ѧʽΪ______��

��1��[Ar]3d6��2�֣� ��2��F��O��As��1�֣�
��3����ad��2�֣�©ѡ��1�֣���ѡ���÷֣� ��sp��1�֣� 1:1��1�֣�
��4��FeF3Ϊ���Ӿ��壬FeCl3Ϊ���Ӿ��壨2�֣��𰸺������ɵ÷֣�
��5����ac��2�֣�©ѡ��1�֣���ѡ���÷֣� ��Ti4CN3��2�֣�
��3����ad��2�֣�©ѡ��1�֣���ѡ���÷֣� ��sp��1�֣� 1:1��1�֣�
��4��FeF3Ϊ���Ӿ��壬FeCl3Ϊ���Ӿ��壨2�֣��𰸺������ɵ÷֣�
��5����ac��2�֣�©ѡ��1�֣���ѡ���÷֣� ��Ti4CN3��2�֣�
�����������1���������ӵĺ����������24����˸��ݺ�����ӵ��Ų����ɿ�֪����̬Fe2+�ĺ�������Ų�ʽΪ[Ar]3d6��
��2���ǽ�����Խǿ���縺��Խ�������Ԫ�������ɿ�֪��������������Ԫ���е縺��ֵ�ɴ�С��˳����F��O��As��
��3����(NH4)3FeF6�����ӻ�������ڵ����������������ۼ�������Ӽ����������λ������N��H��Fe��F֮�������λ������ѡad��
����֪SCNһ�и�ԭ������������8�����ȶ��ṹ����̼Ԫ�طֱ���S�Լ�NԪ���γ�1��˫���������ڹ¶Ե��ӣ����Cԭ�ӵ��ӻ���ʽΪsp�ӻ������ڵ�������
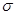 ����˫������1��
����˫������1��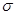 ����1��
����1��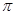 �����ɵģ����ԭ������
�����ɵģ����ԭ������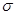 ����
����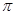 �����ı�ֵΪ1:1��
�����ı�ֵΪ1:1����4��FeCl3����������ˮ���Ҵ����þƾ��Ƽ��ȼ�����������˵���Ȼ����γɵľ����Ƿ��Ӿ��壬��FeF3�����۵����1000oC����˵���������γɵľ������������Ӿ��壬������ֻ������۵����ϴ��ԭ����FeF3Ϊ���Ӿ��壬FeCl3Ϊ���Ӿ��塣
��5����a���ǽ�����Խǿ���⻯����ȶ���Խǿ����������⻯����ȶ������ͣ�a��ȷ��b�����ڰ������Ӽ�����������˰����ķе���ߣ�b����ȷ��c ���ǽ�����Խǿ����Ԫ���γɵĹ��ۼ�Խǿ������Խ�����R��H������ԭ���������������С��c��ȷ��d�������⻯�����ɵľ�����Ƿ��Ӿ��壬���Ӽ�����������Է������������Ӷ�����d����ȷ����ѡac��
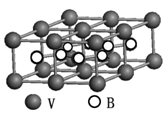
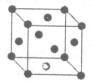
�ڸ��ݾ����Ľṹ�ص㲢���ݾ�̯����֪�������к��е�̼ԭ������8��
 ��1����ԭ����6��
��1����ԭ����6�� ��3����ԭ������12��
��3����ԭ������12�� +1��4�����Ի�ѧʽΪTi4CN3��
+1��4�����Ի�ѧʽΪTi4CN3��
��ϰ��ϵ�д�
�����Ŀ



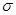 ��
��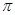 ��
��
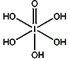 ����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ��:H5IO6_____HIO4 ��������� ������������
����HIO4��ǰ��Ϊ��Ԫ�ᣬ����ΪһԪ�ᡣ��Ƚ϶�������ǿ��:H5IO6_____HIO4 ��������� ������������


 )������˵����ȷ����________(�����)��
)������˵����ȷ����________(�����)��