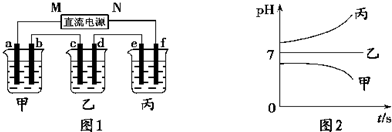
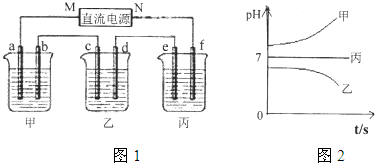
��Ŀ����
��12�֣�A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
| ������ | Na+��K+��Cu2+ |
| ������ | SO42����OH�� |


��ͨ��Դ������һ��ʱ��������c�缫����������16g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ���ϡ��ݴ˻ش��������⣺
��1��MΪ��Դ�� ������д�������������缫b�Ϸ����ĵ缫��ӦΪ ��
��2������缫e�����ɵ������ڱ�״̬�µ������ ��
��3��д�����ձ��ĵ��ط�Ӧ
��4�������������B��Һ�еĽ�������ȫ����������ʱ����ܷ�������У�Ϊʲô��
��5��������һ��ʱ��������c�缫����������16g��Ҫʹ���ָ���ԭ����״̬�������� ��
��1������1�֣�; 4OH����4e��=2H2O + O2����2�֣�
��2��5��6 L��2�֣�
��3��2CuSO4+2H2O 2Cu+O2��+2H2SO4��2�֣�
2Cu+O2��+2H2SO4��2�֣�
��4���ܣ���ΪCuSO4��Һ��ת��ΪH2SO4��Һ����Ӧ��Ϊ���ˮ�ķ�Ӧ��3�֣�
��5������ձ��м�4��5gˮ��2�֣�
����

��ϰ��ϵ�д�
�����Ŀ