��Ŀ����
A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
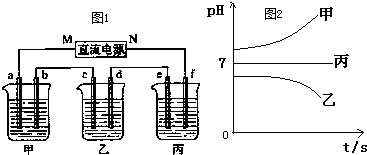
ͼ1��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��������A��Һ��������B��Һ��������C��Һ���缫��Ϊʯī�缫��
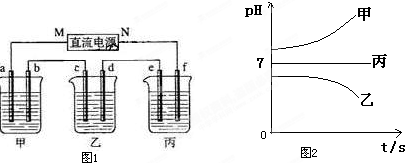
��ͨ��Դ������һ��ʱ��������c�缫����������16g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ��ͼ2���ݴ˻ش��������⣺
��1��MΪ��Դ��
��2��д�����ձ��ĵ�ⷴӦ����ʽ
��3������缫e�����ɵ������ڱ�״̬�µ����
��4��Ҫʹ���ָ���ԭ����״̬����Ҫ����һ��
| ������ | Na+��K+��Cu2+ |
| ������ | SO42-��OH- |

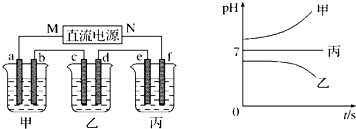
��ͨ��Դ������һ��ʱ��������c�缫����������16g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ��ͼ2���ݴ˻ش��������⣺
��1��MΪ��Դ��
��
��
������д���������������缫b�Ϸ����ĵ缫��ӦΪ4OH--4e-=2H2O+O2��
4OH--4e-=2H2O+O2��
����2��д�����ձ��ĵ�ⷴӦ����ʽ
2CuSO4+2H2O
2Cu+O2��+2H2SO4
| ||
2CuSO4+2H2O
2Cu+O2��+2H2SO4
��
| ||
��3������缫e�����ɵ������ڱ�״̬�µ����
5.6L
5.6L
����4��Ҫʹ���ָ���ԭ����״̬����Ҫ����һ��
ˮ
ˮ
����4.5
4.5
�ˣ���������ͨ��Դ������һ��ʱ��������c�缫����������16g������ӦΪCuSO4��Һ��cΪ������dΪ��������aΪ������bΪ������eΪ������fΪ������MΪ������NΪ�����������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ��ͼ2����pH��������ӦΪ�������ƻ�����������Һ������pH���䣬ӦΪ�����ƻ��������Һ���Դ˽����⣮
����⣺��ͨ��Դ������һ��ʱ��������c�缫����������16g������ӦΪCuSO4��Һ��cΪ������dΪ��������aΪ������bΪ������eΪ������fΪ������MΪ������NΪ�����������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ��ͼ2����pH��������ӦΪ�������ƻ�����������Һ������pH���䣬ӦΪ�����ƻ��������Һ��
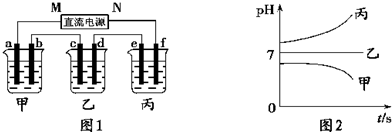
��1�������Ϸ�����֪MΪ��������Ϊ����������ƻ�����������Һ��bΪ����������������Ӧ���缫����ʽΪ4OH--4e-=2H2O+O2����
�ʴ�Ϊ������4OH--4e-=2H2O+O2����
��2����ӦΪCuSO4��Һ�����ʱ����������ͭ������������������ⷽ��ʽΪ2CuSO4+2H2O
2Cu+O2��+2H2SO4��
�ʴ�Ϊ��2CuSO4+2H2O
2Cu+O2��+2H2SO4��
��3��n��Cu��=
=0.25mol��ת�Ƶ���0.5mol����缫e������2H++2e-=H2����������n��H2��=0.25mol��v��H2��=0.25mol��22.4L/mol=5.6L��
�ʴ�Ϊ��5.6L��
��4����Ϊ�����ƻ��������Һ��ʵ����Ϊ���ˮ��ת�Ƶ���0.5molʱ��2H2O
2H2��+O2��������ˮ�����ʵ���Ϊ0.25mol������Ϊ0.25mol��18g/mol=4.5g��
�ʴ�Ϊ��ˮ��4.5��
��1�������Ϸ�����֪MΪ��������Ϊ����������ƻ�����������Һ��bΪ����������������Ӧ���缫����ʽΪ4OH--4e-=2H2O+O2����
�ʴ�Ϊ������4OH--4e-=2H2O+O2����
��2����ӦΪCuSO4��Һ�����ʱ����������ͭ������������������ⷽ��ʽΪ2CuSO4+2H2O
| ||
�ʴ�Ϊ��2CuSO4+2H2O
| ||
��3��n��Cu��=
| 16g |
| 64g/mol |
�ʴ�Ϊ��5.6L��
��4����Ϊ�����ƻ��������Һ��ʵ����Ϊ���ˮ��ת�Ƶ���0.5molʱ��2H2O
| ||
�ʴ�Ϊ��ˮ��4.5��
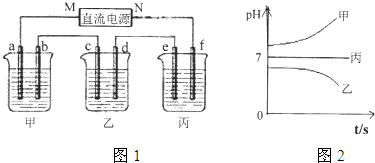
���������⿼����ԭ����������Ĺؼ���c���������仯�Լ�������ҺpH�ı仯��ע��������ӷŵ�˳���յ����ɼ��ɽ����⣬��Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ