��Ŀ����
��2012?ɽ��ģ�⣩A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
��ͼ1��ʾװ���У��ס��ҡ��������ձ����ηֱ�ʢ��������A��Һ��������B��Һ��������C��Һ���缫��Ϊʯī�缫��

��ͨ��Դ������һ��ʱ��������c�缫����������16g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ��ͼ2���ݴ˻ش��������⣺
��1��MΪ��Դ��
�缫b�Ϸ����ĵ缫��ӦΪ
��2������缫e�����ɵ������ڱ�״̬�µ������
��3��д�����ձ��ĵ��ط�Ӧ
��4��������һ��ʱ��������c�缫����������16g��Ҫʹ���ָ���ԭ����״̬�������������
��5�������������B��Һ�еĽ�������ȫ������ʱ������Դ�缫���ӣ����һ��ʱ����ҳ�����Һ��pH
| ������ | Na+��K+��Cu2+ |
| ������ | SO42-��OH- |

��ͨ��Դ������һ��ʱ��������c�缫����������16g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵͼ��ͼ2���ݴ˻ش��������⣺
��1��MΪ��Դ��
��
��
������д�������������缫b�Ϸ����ĵ缫��ӦΪ
4OH--4e-=2H2O+O2��
4OH--4e-=2H2O+O2��
����2������缫e�����ɵ������ڱ�״̬�µ������
5.6L
5.6L
����3��д�����ձ��ĵ��ط�Ӧ
2CuSO4+2H2O
2Cu+O2��+2H2SO4
| ||
2CuSO4+2H2O
2Cu+O2��+2H2SO4
| ||
��4��������һ��ʱ��������c�缫����������16g��Ҫʹ���ָ���ԭ����״̬�������������
CuO
CuO
������20g
20g
����5�������������B��Һ�еĽ�������ȫ������ʱ������Դ�缫���ӣ����һ��ʱ����ҳ�����Һ��pH
����
����
���������С�����䡱�����缫d�ϵķ�Ӧ��Cu2++2e-=Cu
Cu2++2e-=Cu
���������������c�缫����������16g�������к���Cu2+��������ӵĹ����֪��BΪCuSO4������pH���䣬��CΪ�����ƻ�����أ�����pH������AΪKOH��NaOH��
��1������c�缫ͭ���ӵõ��ӣ���cΪ��������MΪ��Դ��������bΪ����������Һ��OH-�ŵ磻
��2��e�缫�������ӷŵ���������������Cu��2e-��H2�������㣻
��3�����ձ���Ϊ���Ե缫�������ͭ��Һ��
��4��Ҫʹ���ָ���ԭ����״̬�����ݵ��ʵ�ʺ����ɲ����Ҫ��������ͭ���������ᷴӦ����������ͭ�����������Cu��H2SO4��CuO���㣻
��5�������������B��Һ�еĽ�������ȫ������ʱ�������Ϊ���ᣬ����Դ�缫���ӣ�ͭ�������ܽ⣬��Һ���������������ŵ磬���缫ͭȫ��ʧȥ���ӣ���ԱPH���䣻
��1������c�缫ͭ���ӵõ��ӣ���cΪ��������MΪ��Դ��������bΪ����������Һ��OH-�ŵ磻
��2��e�缫�������ӷŵ���������������Cu��2e-��H2�������㣻
��3�����ձ���Ϊ���Ե缫�������ͭ��Һ��
��4��Ҫʹ���ָ���ԭ����״̬�����ݵ��ʵ�ʺ����ɲ����Ҫ��������ͭ���������ᷴӦ����������ͭ�����������Cu��H2SO4��CuO���㣻
��5�������������B��Һ�еĽ�������ȫ������ʱ�������Ϊ���ᣬ����Դ�缫���ӣ�ͭ�������ܽ⣬��Һ���������������ŵ磬���缫ͭȫ��ʧȥ���ӣ���ԱPH���䣻
����⣺�������c�缫����������16g�������к���Cu2+��������ӵĹ����֪��BΪCuSO4������pH���䣬��CΪ�����ƻ�����أ�����pH������AΪKOH��NaOH��
��1������c�缫ͭ���ӵõ��ӣ���cΪ��������MΪ��Դ��������bΪ����������Һ��OH-�ŵ磬�缫��ӦΪ4OH--4e-=O2��+2H2O���ʴ�Ϊ������4OH--4e-=O2��+2H2O��
��2��e�缫�������ӷŵ�����������n��Cu��=
=0.25mol����Cu��2e-��H2����֪���ɱ�������������Ϊ0.25mol��22.4L/mol=5.6L���ʴ�Ϊ��5.6L��
��3�����ձ���Ϊ���Ե缫�������ͭ��Һ���ܷ�ӦΪ2CuSO4+2H2O
2Cu+O2��+2H2SO4��
�ʴ�Ϊ��2CuSO4+2H2O
2Cu+O2��+2H2SO4��
��4��������һ��ʱ��������c�缫����������16g��Ҫʹ���ָ���ԭ����״̬�����ݵ�ⷴӦ2CuSO4+2H2O
2Cu+O2��+2H2SO4����Ҫ����CuO�����ɵ����ᷴӦ��������ͭ��ˮ���õ�Cu��H2SO4��CuO��n��Cu��=
=0.25mol��n��CuO��=0.25mol����Ҫ��������ͭ����=0.25mol��80g/mol=20g��
�ʴ�Ϊ��CuO��20g��
��5�������������B��Һ�еĽ�������ȫ������ʱ�������Ϊ���ᣬ����Դ�缫���ӣ�ͭ�������ܽ⣬��Һ���������������ŵ磬���缫ͭȫ��ʧȥ���ӣ���ԱPH���䣻�缫��ӦΪCu2++2e-=Cu��
�ʴ�Ϊ�����䣻 Cu2++2e-=Cu��
��1������c�缫ͭ���ӵõ��ӣ���cΪ��������MΪ��Դ��������bΪ����������Һ��OH-�ŵ磬�缫��ӦΪ4OH--4e-=O2��+2H2O���ʴ�Ϊ������4OH--4e-=O2��+2H2O��
��2��e�缫�������ӷŵ�����������n��Cu��=
| 16g |
| 64g/mol |
��3�����ձ���Ϊ���Ե缫�������ͭ��Һ���ܷ�ӦΪ2CuSO4+2H2O
| ||
�ʴ�Ϊ��2CuSO4+2H2O
| ||
��4��������һ��ʱ��������c�缫����������16g��Ҫʹ���ָ���ԭ����״̬�����ݵ�ⷴӦ2CuSO4+2H2O
| ||
| 16g |
| 64g/mol |
�ʴ�Ϊ��CuO��20g��
��5�������������B��Һ�еĽ�������ȫ������ʱ�������Ϊ���ᣬ����Դ�缫���ӣ�ͭ�������ܽ⣬��Һ���������������ŵ磬���缫ͭȫ��ʧȥ���ӣ���ԱPH���䣻�缫��ӦΪCu2++2e-=Cu��
�ʴ�Ϊ�����䣻 Cu2++2e-=Cu��
���������⿼����ԭ������ȷ�����ĵ缫��Ӧ����ⷴӦ��ͼ��ķ����ǽ����Ĺؼ���ע���������Ƴ������ʼ���Դ���������ǽ���ͻ�ƿڣ���Ŀ�Ѷ��е�

��ϰ��ϵ�д�
 �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
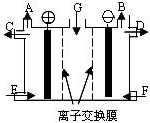 ��2012?ɽ��ģ�⣩���������ӽ���Ĥ��ֻ������Ӧ������ͨ������ʯī���缫�ĵ��۵�ⱥ�͵�Na2SO4��Һ����NaOH��H2SO4������˵������ȷ���ǣ�������
��2012?ɽ��ģ�⣩���������ӽ���Ĥ��ֻ������Ӧ������ͨ������ʯī���缫�ĵ��۵�ⱥ�͵�Na2SO4��Һ����NaOH��H2SO4������˵������ȷ���ǣ�������