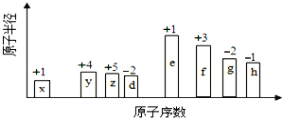
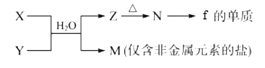��Ŀ����
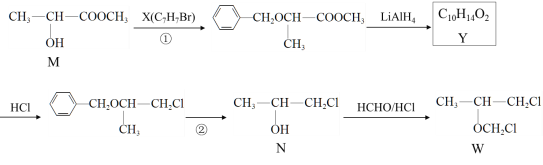
����Ŀ����Τ��(TDF)��Ҫ�������ư��̲���(HIV)��Ⱦ��������W�Ǻϳ�TDF����Ҫ�м��壬��ϳ�·����ͼ��ʾ��
���������գ�
(1)N���������ŵ�������______________��Y�Ľṹ��ʽ��____________________��
(2)X���ñ���һ��ͬϵ���Ʊ�����������Լ���������____________________��
(3)д������W���Ƿ��в�����ȩ�Ļ�ѧ����ʽ____________________________________��
(4)�٢ڵ�Ŀ����_______________________��
(5)д��M��������������һ��ͬ���칹��Ľṹ��ʽ��_______________
i. ��M������ͬ������ ii. �ܷ���������Ӧ
(6)д����MΪԭ���Ʊ��۱�ϩ��( )�ĺϳ�·��_______________________ ��
)�ĺϳ�·��_______________________ ��
���𰸡��ǻ�����ԭ�� 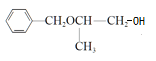 Br2 ���� HCHO + 4Cu(OH)2
Br2 ���� HCHO + 4Cu(OH)2 ![]() 2Cu2O�� + CO2 ��+ 5H2O �����ǻ���ʹ�䲻��HCl����ȡ�� HCOOCH2CH2CH2OH
2Cu2O�� + CO2 ��+ 5H2O �����ǻ���ʹ�䲻��HCl����ȡ�� HCOOCH2CH2CH2OH ![]()
![]()
��������
��1��![]() ���������ŵ������ǣ�-OH���ǻ��ͣ�-Cl����ԭ�ӡ�
���������ŵ������ǣ�-OH���ǻ��ͣ�-Cl����ԭ�ӡ�
��2��X��C7H7Br�����ɼױ���Br2�ڹ���������ȡ�����ϵ���ԭ���Ƶá�
��3������ȩ���ķ����������Ƶ�Cu(OH)2����Һ�����ȣ���ש��ɫ�������ɵ�˵������ȩ����
��4���٢ڵ�Ŀ���DZ����ǻ���ʹ�䲻��HCl����ȡ����
��5����M������ͬ������ ��˵�������������ǻ������� ���ܷ���������Ӧ ��˵����ȩ����
��6����MΪԭ���Ʊ��۱�ϩ��(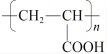 )�ĺϳ�·��Ϊ��
)�ĺϳ�·��Ϊ��
![]()
![]() ��
��
��1�� ![]() �����������ŵ������ǣ�-OH���ǻ��ͣ�-Cl����ԭ�ӣ�����
�����������ŵ������ǣ�-OH���ǻ��ͣ�-Cl����ԭ�ӣ�����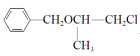 ���Ƴ�Y�Ľṹ��ʽ��
���Ƴ�Y�Ľṹ��ʽ��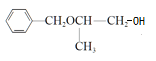 ���ʴ�Ϊ���ǻ�����ԭ�ӣ�
���ʴ�Ϊ���ǻ�����ԭ�ӣ�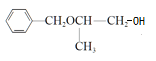 ��
��
��2��X��C7H7Br�����ɼױ���Br2�ڹ���������ȡ�����ϵ���ԭ���Ƶã��ʴ�Ϊ��Br2 �����ա�
��3������ȩ���ķ����������Ƶ�Cu(OH)2����Һ�����ȣ���ש��ɫ�������ɵ�˵������ȩ������Ӧ�ķ���ʽΪ��HCHO + 4Cu(OH)2 ![]() 2Cu2O�� + CO2 ��+ 5H2O���ʴ�Ϊ��HCHO + 4Cu(OH)2
2Cu2O�� + CO2 ��+ 5H2O���ʴ�Ϊ��HCHO + 4Cu(OH)2 ![]() 2Cu2O�� + CO2 ��+ 5H2O��
2Cu2O�� + CO2 ��+ 5H2O��
��4���٢ڵ�Ŀ���DZ����ǻ���ʹ�䲻��HCl����ȡ�����ʴ�Ϊ�������ǻ���ʹ�䲻��HCl����ȡ����
��5��MΪ![]() ����M������ͬ������ ��˵�������������ǻ������� ���ܷ���������Ӧ ��˵����ȩ���� �ʷ���������ͬ���칹����HCOOCH2CH2CH2OH���ʴ�Ϊ��HCOOCH2CH2CH2OH��
����M������ͬ������ ��˵�������������ǻ������� ���ܷ���������Ӧ ��˵����ȩ���� �ʷ���������ͬ���칹����HCOOCH2CH2CH2OH���ʴ�Ϊ��HCOOCH2CH2CH2OH��
��6����MΪԭ���Ʊ��۱�ϩ��(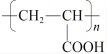 )�ĺϳ�·��Ϊ��
)�ĺϳ�·��Ϊ��
![]()
![]() ���ʴ�Ϊ��
���ʴ�Ϊ��
![]()
![]() ��
��

����Ŀ��������ˮ����ͨ�����軯�صı������ᷢ����Ӧ��4KSCN + 9H2O(g) �� K2CO3 + K2S + 3CO2 + 3H2S + 4NH3���������գ�
(1)������Ӧ���漰�ĸ�Ԫ���У����Ӱ뾶������_____________(�����ӷ���)�����ڵڶ�����Ԫ�صķǽ�������ǿ������˳��Ϊ_________________��
(2)������Ӧ���漰�ĸ������У����ڷǵ���ʵ���_________________��
(3)д��CO2�ĵ���ʽ____________�����۷е�͵�ԭ����___________________________��
(4)��֪���ʵ���Ũ�Ⱦ�Ϊ0.10 mol/L��K2CO3��K2S��ҺpH���£�
��Һ | K2CO3 | K2S |
pH | 11.6 | 12.5 |
K2CO3ˮ��Һ�ʼ��Ե�ԭ����______________________________(�����ӷ���ʽ��ʾ)���ӱ������ݿ�֪��K2CO3��Һ��c(CO32-)_______K2S��Һ�е�c(S2��)(������������������=��)��
(5)K2S�Ӵ���ʪ�������棬����ֺ�ɫ�ߵ�(Ag2S)����ԭ�����£�____K2S + ____Ag + _____O2 + _____H2O �� _____Ag2S + _____KOH
����ƽ������Ӧ________________��
��ÿ���ı�״����224 mL O2��ת�Ƶ�����ĿΪ_____________��
����Ŀ��Na2SO3�dz�����һ���Σ���ҵ�Ͽ�����������ȥ�ȼ��ͻ�ԭ����
(1)Na2SO3�������������ǿ�ȣ���Ӧ��Ĺ����к���S2-����Ӧ�Ļ�ѧ����ʽΪ________________������0.5molNa2SO3�μӷ�Ӧ�������ת�Ƹ���Ϊ_____________________��
(2)��0.1mol/L Na2SO3��Һ�������ٽ��£��ⶨ�¶ȱ仯�����е�pH���������£�
ʱ�� | �� | �� | �� | �� |
�¶�/�� | 25 | 30 | 40 | 25 |
pH | 9.66 | 9.52 | 9.37 | 9.25 |
��ʱ��Na2SO3��Һ��ˮ�ĵ���̶�_______ͬ���´�ˮ��ˮ�ĵ���̶�(��)��>������<������=��)��Ӧ��ƽ��ԭ������ԭ��_________________________________���ܵ�pH��С�ڢ٣�������______________________�����һ����ʵ����֤��_____________________��
(3)��Na2SO3��Һ����������Ũ���ɴ�С����_________________________________��
����Ŀ���¶�ΪTʱ����2.0L�����ܱ������г���1.0 mol PCl5����ӦPCl5(g)![]() PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±�������˵����ȷ����
PCl3(g)��Cl2(g)��һ��ʱ���ﵽƽ�⡣��Ӧ�����вⶨ�IJ������ݼ��±�������˵����ȷ����
t/s | 0 | 50 | 150 | 250 | 350 |
n(PCl3)/ mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
A����Ӧ��ǰ50 s��ƽ������Ϊv(PCl3)=0.0032mol��L��1��s��1
B�����������������䣬�����¶ȣ�ƽ��ʱc(PCl3)=0.11mol��L��1����Ӧ����H��0
C����ͬ�¶��£���ʼʱ�������г���1.0mol PCl5��0.20mol PCl3��0.20molCl2���ﵽƽ��ǰv(��)��v(��)
D����ͬ�¶��£���ʼʱ�������г���2.0mol PCl3��2.0molCl2���ﵽƽ��ʱ��PCl3��ת����С��80%