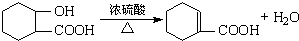
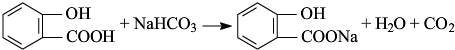
��Ŀ����
2����Ҫ�����������գ���1����0.2mol��A����������ȫȼ��ʱ����CO2��H2O��1.2mol�������������2��2-�������飬��A�Ľṹ��ʽΪ��CH3��3C-CH=CH2��
��2��ijȲ����H2��ּӳ�����2��5-�������飬���Ȳ���Ľṹ��ʽΪ��CH3��2CH-C��C-CH��CH3��2��
��3��ij��̬��100mL����״�����뺬1.43g�����ˮǡ����ȫ��Ӧ�����ⶨ��������ÿ��̼ԭ���϶���1����ԭ�ӣ�������Ľṹ��ʽΪCH2=CH-CH=CH2��
��4����Է�������Ϊ84��������������������һ�ȴ���ֻ��3�֣����ķ���ʽΪC6H12��д�����������нṹ��ʽ��CH3CH2CH=CHCH2CH3��CH3CH2��CH3CH2��C=CH2��
���� ��1������CԪ�ء�HԪ���غ�ȷ�������ķ���ʽΪC6H12���ڴ�����������H2�����ӳɷ�Ӧ������2��2-�������飬������Ľṹ��ʽΪ��CH3��3C-CH=CH2��
��2������2��5-��������Ľṹ��֪��Ȳ���еIJμ�ֻ����3��̼��4��̼֮�䣬�ݴ�ȷ��Ȳ�Ľṹ��ʽ��
��3������n=$\frac{V}{{V}_{m}}$ȷ�����������ʵ���������n=$\frac{m}{n}$�����������ʵ�����Ȼ�����ÿ��̼ԭ���϶���1����ԭ��ȷ�������ķ���ʽ���Ӷ�ȷ�������Ľṹ��ʽ��
��4���������෨ȷ�������ķ���ʽ��Ȼ����������������д������Ľṹ��ʽ��
��� �⣺��1��n��������n��C����n��H��=n��������n��CO2����2n��H2O��=0.1mol��0.6mol��0.6mol��2=1��6��12����1�������к���6��Cԭ�ӡ�12��Hԭ�ӣ��ʸ����ķ���ʽΪC6H12���ڴ�����������H2�����ӳɷ�Ӧ������2.2-�������飬������Ľṹ��ʽΪ����CH3��3C-CH=CH2��
�ʴ�Ϊ����CH3��3C-CH=CH2��
��2������2��5-��������Ľṹ��֪��Ȳ����H2��ּӳ�����2��5-�������飬Ȳ���еIJμ�ֻ����3��̼��4��̼֮�䣬����Ȳ�Ľṹ��ʽΪ����CH3��2CH-C��C-CH��CH3��2��
�ʴ�Ϊ����CH3��2CH-C��C-CH��CH3��2��
��3�������100mL���������ʵ���Ϊ��$\frac{0.1L}{22.4L/mol}$=$\frac{1}{224}$mol��
1.43gBr2�����ʵ���Ϊ��$\frac{1.43g}{160g/mol}$=$\frac{143}{16000}$mol������Brԭ�ӵ����ʵ���Ϊ��$\frac{143}{8000}$mol��
ÿ��C����һ����ԭ�ӣ�������x��̼����$\frac{1}{224}$mol��x=$\frac{143}{8000}$mol��
��ã�x=4�������к��е�̼ԭ�Ӹ���Ϊ4��
�����Ľṹ��ʽΪ��CH2=CH-CH=CH2��
�ʴ�Ϊ��CH2=CH-CH=CH2��
��4���л������Է�������Ϊ84���ҽ�����̼��������Ԫ�أ��������෨$\frac{84}{14}$=6�������ķ���ʽΪ��C6H12������Ϊ��������������к���̼̼˫������������������һ�ȴ���ֻ��3�֣�A�����к�����������������к���3�ֵ�ЧH�������Ľṹ��ʽΪ��CH3CH2CH=CHCH2CH3��CH3CH2��CH3CH2��C=CH2��
�ʴ�Ϊ��C6H12��CH3CH2CH=CHCH2CH3��CH3CH2��CH3CH2��C=CH2��
���� ���⿼�����л������ʽ���ṹ��ʽ��ȷ������Ŀ�Ѷ��еȣ���ȷ�����л���ṹ������Ϊ���ؼ���ע������ͬ���칹��ĸ����дԭ������������ѧ�����Ӧ�û���֪ʶ��������

 �ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�
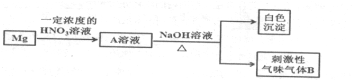
�ϴ�̸�������������νӽ̳��Ͼ���ѧ������ϵ�д�| A�� | SO2 | B�� | H2S | C�� | BBr3 | D�� | COCl2 |
| A�� | ���ϣ�������ۡ���ϳɷ������ۡ����ػ�����л�������ṹ����ά��ʶ��Ϊ�л���ѧ�ķ�չ�������� | |
| B�� | ͬλ��ʾ�ٷ����˴Ź���������������������о��л���Ӧ��������Ҫ���� | |
| C�� | ϴ����������ȡ����Һ���ᾧ�����л�������ķ������ᴿ�ij������� | |
| D�� | �¹���ѧ��ά�����Ʊ������ʱ�õ������أ�������������л���Ľ��� |
| W | X | |
| Y | Z |
| A�� | Y����̬�⻯�����ȶ� | B�� | Z�ĵ��������ӻ�ԭ����ǿ | ||
| C�� | X���ʳ����»�ѧ���ʻ��� | D�� | Y��ԭ��������W��7 |
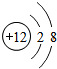 ��
�� ��������������һϵ�����ʣ��йر仯��ͼ��
��������������һϵ�����ʣ��йر仯��ͼ��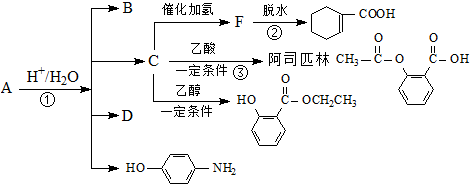
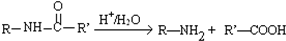
 ��
�� ��
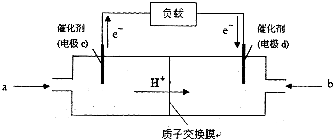
��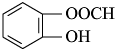 ����������
�����λ���λ����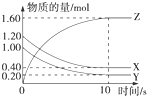 ��1��ij�¶��£�2L�����ܱ������У�X��Y��Z�������巢����ѧ��Ӧʱ�����ʵ�����ʱ��仯�Ĺ�ϵ������ͼ��ʾ����
��1��ij�¶��£�2L�����ܱ������У�X��Y��Z�������巢����ѧ��Ӧʱ�����ʵ�����ʱ��仯�Ĺ�ϵ������ͼ��ʾ����