��Ŀ����
����������ȷ����
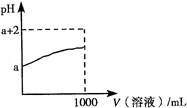
A������ʱ��ij��Һ����ˮ���������c��H������c��OH�����ij˻�Ϊ1��10��24������Һ��һ�����Դ�������K����Na����AlO2����SO42��
B������ʱ��0.1mol/L HA��Һ��pH��1��0.1mol/L BOH��Һ��c��OH����/c��H����=1012������������Һ�������ϣ���Ϻ���Һ������Ũ�ȵĴ�С��ϵΪ��
c��B������c��OH������c��H������c��A����
C������SO2ͨ�뵽Ba��NO3��2��Һ�У���ȷ�����ӷ�Ӧ����ʽΪ��
3SO2��2NO3����3Ba2����2H2O=3BaSO4����2NO����4H��
D����pH=2��pH=3�Ĵ�����Һ�кͺ�����NaOH����Һ�����ĵĴ�����Һ������ֱ�ΪVa��Vb����Vb<10Va
C
����:��Һ����ˮ���������c��H������c��OH�����ij˻�Ϊ1��10��24��˵��ˮ�ĵ��뱻�����ˣ�����������������ƣ�������Һ����������Ҳ�����Լ��ԣ�AlO2����������Һ�в��ܴ������棬A����ȷ��0.1mol/L HA��Һ��pH��1��˵��HA�����ᣬ0.1mol/L BOH��Һ��c��OH����/c��H����=1012����������ˮ�����ӻ����������c��OH������0.1mol/L�����Լ���ǿ���Ӧ����Һ���ɵ���ABˮ���Լ��ԣ�����ˮ��̶�һ�㶼�Ƚ�С����������Ũ�ȵĴ�С˳��Ϊc��B������c��A������c��OH������c��H������B����ȷ��SO2���л�ԭ�ԣ�����ˮ�����ԣ�NO3�������������������������ᱵ������C��ȷ����pH=2��pH=3�Ĵ����Ũ�ȷֱ���x��y����Ϊ�����������ᣬ����pH=2�Ĵ���ϡ��10������Һ��pHӦС��3�����Ҫϡ�͵�pH=3������Ҫ������ˮ������y��x/10����x��10y����Vb��10Va��D����ȷ����ѡC��

 ��У����ϵ�д�
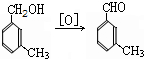
��У����ϵ�д�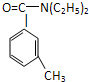 ���ð�������DEET����һ�ֶ��˰�ȫ�����Ը�����ҩ�Ե��������ü�����ṹ��ʽΪ����֪��RCOOH
���ð�������DEET����һ�ֶ��˰�ȫ�����Ը�����ҩ�Ե��������ü�����ṹ��ʽΪ����֪��RCOOH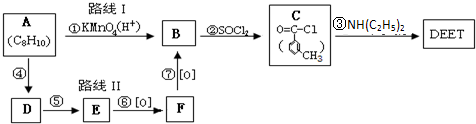
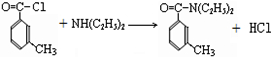
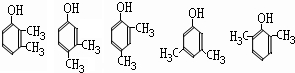 ����д2�֣�
����д2�֣�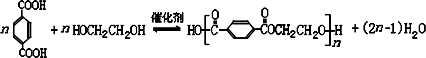
 NH3?H2O+H+
NH3?H2O+H+ ��2010?��������ģ��X��Y��Z��W��Ϊ����10���ӵ���������X��Y��ZΪ���ӣ�WΪ���ӣ���X��Z�����к��еĹ��õ��Ӷ���֮��Ϊ3��4��
��2010?��������ģ��X��Y��Z��W��Ϊ����10���ӵ���������X��Y��ZΪ���ӣ�WΪ���ӣ���X��Z�����к��еĹ��õ��Ӷ���֮��Ϊ3��4��