��Ŀ����
����Ŀ�����ȷ���(H)��һ�����͡�ǿЧ���ȡ���ʹ�����ؽ���ҩ���ϳ�·�����£����ַ�Ӧ����ʡ�ԣ���

��֪��

��ش�
��1��������F�Ľṹ��ʽ��__________________��
��2��д��C+D��E�Ļ�ѧ����ʽ____________________________________________��
��3������˵������ȷ����__________��
A��������A������ԭ�ӿ϶�����ͬһƽ��
B��������B�����
C��������E��NaOH��Һ��Ӧ�������4molNaOH
D�����ȷ������ʽΪC16H12Cl2NO4
��4����֪C��ͬ���칹���ж��֣�д��ͬʱ������������������ͬ���칹��Ľṹ��ʽ_______��
��IR�ױ��������к������������ṹ����2����ԭ�Ӳ���ͬһ�������ϣ�
��1H-NMR����ʾֻ��5�ֲ�ͬ��������ԭ��
��5������Ա�����ϩΪԭ�Ϻϳ� ![]() ��·��(������ͼ��ʾ�����Լ���ѡ)��
��·��(������ͼ��ʾ�����Լ���ѡ)��
______________________________________________________________________________
���𰸡�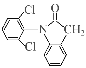
 CD
CD ![]()
![]()
��������
����E�Ľṹ��ʽ������Ӧ���̿�֪��A���б�������������ԭ���ڼ�λ����ṹ��ʽΪ��![]() ��ͬ����B�Ľṹ��ʽΪ��
��ͬ����B�Ľṹ��ʽΪ��![]() ��A��B��Ӧ����C����C�Ľṹ��ʽΪ��
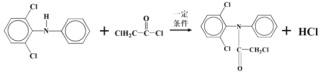
��A��B��Ӧ����C����C�Ľṹ��ʽΪ��![]() ��C��D��Ӧ����E�����Ʋ�D�Ľṹ��ʽΪ��ClCOCH2Cl��ͨ��G��E�Ľṹ��ʽ�ĶԱȣ���Ϸ�Ӧ��֪F�Ľṹ��ʽΪ��
��C��D��Ӧ����E�����Ʋ�D�Ľṹ��ʽΪ��ClCOCH2Cl��ͨ��G��E�Ľṹ��ʽ�ĶԱȣ���Ϸ�Ӧ��֪F�Ľṹ��ʽΪ�� ��
��
��1�����ݷ�����֪��F�Ľṹ��ʽΪ�� ��
��
��2��C�Ľṹ��ʽΪ��![]() �� D�Ľṹ��ʽΪ��ClCOCH2Cl��C��D��Ӧ����E�����ݷ�Ӧ�ڵ��ص㣬��Ӧ�ķ���ʽΪ��
�� D�Ľṹ��ʽΪ��ClCOCH2Cl��C��D��Ӧ����E�����ݷ�Ӧ�ڵ��ص㣬��Ӧ�ķ���ʽΪ��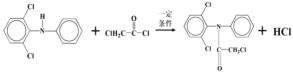 ��
��
��3��A.������A�Ľṹ��ʽΪ��![]() ��Clԭ��ȡ�������ϵ��⣬������ԭ�ӹ�ƽ�棬A��ȷ��
��Clԭ��ȡ�������ϵ��⣬������ԭ�ӹ�ƽ�棬A��ȷ��
B.������B�Ľṹ��ʽ��![]() �����а����������Լ��ԣ�B��ȷ��
�����а����������Լ��ԣ�B��ȷ��
C.������EΪ��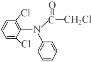 ���ļ��ڼ���������ˮ�⣬Clԭ��ˮ�⣬�����ϵ�Clԭ��ˮ������ɷ��ǻ�����Ӧ����6molNaOH��C����
���ļ��ڼ���������ˮ�⣬Clԭ��ˮ�⣬�����ϵ�Clԭ��ˮ������ɷ��ǻ�����Ӧ����6molNaOH��C����
D. ���ȷ������ʽΪC16H13Cl2NO4��D����
��ΪCD
��4��C�ķ���ʽΪ��C12H9NCl2����IR�ױ��������к������������ṹ����2����ԭ�Ӳ���ͬһ�������Ϣ�1H-NMR����ʾֻ��5�ֲ�ͬ��������ԭ�ӣ�����������Ϊ�Գƽṹ�����ܵĽṹ��ʽΪ��![]() ��
��
��5�����ݷ�Ӧ�ٿ�֪������������֧������![]() ������
������![]() ������Ҫ�ȴ������軯�������������·�Ӧ��ȡ���ʿ�������ϩ��ȡ�ȴ����������軯�Ʒ�Ӧ���ɣ�����Ϊ��
������Ҫ�ȴ������軯�������������·�Ӧ��ȡ���ʿ�������ϩ��ȡ�ȴ����������軯�Ʒ�Ӧ���ɣ�����Ϊ��![]() ��
��

����Ŀ��һ�������£�ͨ�����з�Ӧ�����Ʊ������մɵ�ԭ��MgO�� MgSO3(s) + CO(g)![]() MgO(s) + CO2(g) +SO2(g) ��H>0���÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ�������
MgO(s) + CO2(g) +SO2(g) ��H>0���÷�Ӧ�ں��ݵ��ܱ������дﵽƽ��������ı�ͼ�к�����x��ֵ�����´ﵽƽ���������y��x�仯���ƺ�������

ѡ�� | x | y |
A | �¶� | �����ڻ��������ܶ� |
B | CO�����ʵ��� | CO2��CO�����ʵ���֮�� |
C | SO2��Ũ�� | ƽ�ⳣ��K |
D | MgSO4����������������� | CO��ת���� |
����Ŀ�����Ļ�������;�㷺���ش��������⣺
��1����һ�������£������ܺ�ˮ������Ӧ���ɰ���������2N2��g����6H2O��g��=4NH3��g����3O2��g����H����÷�Ӧ��صĻ�ѧ�������������£�
��ѧ�� | N��N | H��O | N��H | O=O |
E��kJ/mol�� | 946 | 463 | 391 | 496 |
��÷�Ӧ����H��________kJ��mol-1��
��2���ں����ܱ������г���2 mol N2O5��1molO2������Ӧ4NO2 (g) + O2 (g) ![]() 2N2O5 (g) ��H��
2N2O5 (g) ��H��
����֪�ڲ�ͬ�¶��²��N2O5�����ʵ�����ʱ��ı仯��ͼ��ʾ���÷�Ӧ����H_____0���������������������������������¸÷�Ӧ�������Է����У���ԭ����___________________��

�������йظ÷�Ӧ��˵����ȷ����_______�����ţ���
A���������������ƽ�����淴Ӧ�����ƶ������������ɫ����
B�����º��ݣ��ٳ���2 mol NO2��1molO2���ٴδﵽƽ��ʱ��NO2��ת��������
C�����º��ݣ��������ڵ��ܶȱ��ֲ���ʱ����Ӧ�ﵽ��ƽ��״̬
D�����÷�Ӧ��ƽ�ⳣ��������һ���ǽ������¶�
��3��N2O5��һ��������ɫ�����������Ʊ����������⻯��ȼ�ϵ������Դ�����õ�ⷨ�Ʊ��õ�N2O5������ԭ����ͼ��ʾ�������⻯��ȼ�ϵ�صĸ�����ӦʽΪ_________��

��4��X��Y��Z��W�ֱ���HNO3��NH4NO3��NaOH��NaNO2����ǿ������е�һ�֡��±��dz�����Ũ�Ⱦ�Ϊ0��01molL��1��X��Y��Z��W��Һ��pH����X��Y��Z��1molͬʱ����ˮ�еõ������Һ��������Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ________��
0��01molL��1����Һ | X | Y | Z | W |
pH | 12 | 2 | 8.5 | 4.5 |
��5�������������������ڴ����еĺ������������ʱ���漰���·�Ӧ��
I��2NO2(g)+NaCl(s) ![]() NaNO3 (s)+ClNO(g) K1
NaNO3 (s)+ClNO(g) K1
��2NO(g)+Cl2 (g) ![]() 2CNO(g) K2
2CNO(g) K2
��4NO2��g����2NaCl��s��![]() 2NaNO3��s����2NO��g����Cl2��g����ƽ�ⳣ��K��____����K1��K2��ʾ����
2NaNO3��s����2NO��g����Cl2��g����ƽ�ⳣ��K��____����K1��K2��ʾ����
���ں��������£���2L�����ܱ������м���0��2 mol NO��0��1 mol Cl2��10minʱ��Ӧ��ﵽƽ�⣬���10min��v(ClNO)��7.5��10-3molL-1min-1����ƽ��ʱNO��ת������1=____���������������䣬��Ӧ���ں�ѹ�����½��У�ƽ��ʱNO��ת������2__��1����������������������������
����Ŀ����100mLij�����м����Ͼ��ȵ�NaHCO3��KHCO3�����ĩ����ַ�Ӧ��ʹ����ȫ���ݳ��������������������������״���£��Ĺ�ϵ���±���
��������/g | 11.25 | 45.00 | >45.00 |
�������/L | 2.80 | 11.2 | 11.2 |
��ش�
��1����������ʵ���Ũ����_____��
��2���������ĩ������Ϊx g������������Ϊ140mL��������CO2���ΪyL����״���£�����д����ĩ����x(g)�����CO2���y(L)֮��Ĺ�ϵ��______��



