题目内容
【题目】某化学兴趣小组拟探究锡及锡的化合物的部分性质。经查阅资料知:Sn的熔点231℃;SnCl2易被氧化,且易水解;Sn(OH)2常温下易分解,SnCl4常温下为无色液体,熔点-33℃,沸点114.1℃,请回答下列问题:
(1)该小组用以下流程制备SnSO4晶体:

①操作Ⅱ所需的实验仪器为______________________________________________。
②过滤操作中玻璃棒使用的注意事项为____________________________。
③操作Ⅰ为沉淀的洗涤。请简述如何判断沉淀是否洗涤干净:____________________。
(2)用熔融的锡与干燥的氯气制备SnCl4,提供的装置如下:

①装置Ⅲ为冷凝管,则水流从________进入。
②请用大写英文字母按从左到右顺序连接组装仪器_________________________________________。
③有同学指出②中连接的实验装置存在不足,不足之处为______________________。
(3)测定锡粉质量分数的步骤:取锡粉1.226g溶于盐酸中,加入过量的FeCl3溶液,再用0.1000mol·L1 K2Cr2O7溶液滴定Fe2+,消耗K2Cr2O7溶液32.00mL,发生的反应:6FeCl2+K2Cr2O7+
14HCl===6FeCl3+2KCl+2CrCl3+7H2O。则锡粉的质量分数为(杂质不参与反应)____________。
【答案】(1)①蒸发皿、玻璃棒、酒精灯、三脚架(或带铁圈的铁架台)、火柴;②玻瑞棒有一定倾斜角;引流时烧怀尖嘴紧靠在玻璃棒上;玻瑞棒未端轻靠在多层滤纸上;③取洗涤液少许于试管中.滴入用HNO3酸化的AgNO3溶液.若无明显.理象.则证明洗涤干净;否则洗涤不干净;(2)①Q;②BJIKACDGHE(F);③缺少尾气处理装置(3)93.18%
【解析】
试题(1)①从溶液中获得溶质的晶体,可以通过蒸发结晶得到,因此操作Ⅱ所需的实验仪器为蒸发皿、玻璃棒、酒精灯、三脚架(或带铁圈的铁架台)、火柴,故答案为:蒸发皿、玻璃棒、酒精灯、三脚架(或带铁圈的铁架台)、火柴;
②过滤操作中使用玻璃棒时要注意:玻瑞棒有一定倾斜角;引流时烧怀尖嘴紧靠在玻璃棒上;玻瑞棒未端轻靠在多层滤纸上,故答案为:玻瑞棒有一定倾斜角;引流时烧怀尖嘴紧靠在玻璃棒上;玻瑞棒未端轻靠在多层滤纸上;
③操作I前的过滤,得到的滤液中含有氯离子,判断沉淀是否洗涤干净,只需判断沉淀上是否还吸附有氯离子即可,方法是取洗涤液少许于试管中.滴入用HNO3酸化的AgNO3溶液.若无明显.理象.则证明洗涤干净;否则洗涤不干净,故答案为:取洗涤液少许于试管中.滴入用HNO3酸化的AgNO3溶液.若无明显.理象.则证明洗涤干净;否则洗涤不干净;
(2)①使用冷凝管时,冷却水应该下进上出,水流从Q进入,故答案为:Q;
②用熔融的锡与干燥的氯气制备SnCl4,首先要制备干燥的氯气,应该选择装置Ⅱ和Ⅵ,且先除氯化氢,再干燥;然后将干燥的氯气通入Ⅰ中反应生成SnCl4,根据SnCl4的物理性质可知,SnCl4的沸点较低,可以用Ⅲ使之冷凝,用Ⅴ收集生成的SnCl4,为了防止外界水蒸气等进入装置,最后连接一个干燥装置Ⅳ,从左到右组装仪器的顺序为BJIKACDGHE(F),故答案为:BJIKACDGHE(F);
③反应中的氯气可能不完全反应,会造成空气污染,故答案为:缺少尾气处理装置;
(3)由Sn+2HCl→SnCl2+H2↑①,
SnCl2+2FeCl3=SnCl4+2FeCl2②,
6FeCl2+K2Cr2O7+14HCl→6FeCl3+2KCl+ 2CrCl3+7H2O,6Sn~K2Cr2O7③
由方程式①②③得知K2Cr2O7~6FeCl2~3SnCl2~3Sn,
n(Sn)=3n(K2Cr2O7)=3×0.1000mol/L×0.032L=0.0096mol,
m(Sn)="n(Sn)×M(Sn)=" 0.0096mol×119g/mol= 1.1424g,
锡粉样品中锡的质量分数=![]() ×100%=
×100%=![]() ×100% =93.18%,故答案为:93.18%。
×100% =93.18%,故答案为:93.18%。

 暑假作业暑假快乐练西安出版社系列答案
暑假作业暑假快乐练西安出版社系列答案【题目】某温度下,在甲、乙、丙、丁四个恒容密闭容器中投入H2和I2,发生反应:H2(g)+I2(g) ![]() 2HI(g)。反应体系中各物质浓度的有关数据如下。
2HI(g)。反应体系中各物质浓度的有关数据如下。
容器 | 起始浓度 | 平衡浓度 | |
c(H2)/(mol·L-1) | c(I2)/(mol·L-1) | c(HI)/(mol·L-1) | |
甲 | 0.01 | 0.01 | 0.004 |
乙 | 0.01 | 0.02 | a |
丙 | 0.02 | 0.01 | b |
丁 | 0.02 | 0.02 | c |
下列判断不正确的是
A. HI的平衡浓度:a=b>0.004,c=0.008 B. 平衡时,H2的转化率:丁>甲
C. 平衡时,乙中H2的转化率大于20% D. 丙中条件下,该反应的平衡常数K=0.25
【题目】CO、H2、CH3OH均是清洁能源。
(1)已知部分化学键键能数据如下:
化学键 | C | O=O | C=O | C-O |
E/(kJ mol-1) | 958.5 | 497 | 745 | 351 |
2CO(g) +O2(g)==2CO2(g) ![]() H1 H2O(g)+CO(g)==H2(g) + CO2(g)
H1 H2O(g)+CO(g)==H2(g) + CO2(g) ![]() H2 = -41 kJmol-1
H2 = -41 kJmol-1
CH3OH(g)+ 3/2O2(g)==CO2(g)+2H2O(g) ![]() H3 = -660kJmol-1
H3 = -660kJmol-1
则△H1=_____ kJmol-1,反应CO(g)+2H2(g)![]() CH3OH(g)的△H=_____ kJmol-1。
CH3OH(g)的△H=_____ kJmol-1。
(2)一定条件下,在容积为2 L的密闭容器Q中充入a mol CO与b molH2合成甲醇:CO(g) +2H2(g) ![]() CH3OH(g)。测得平衡时混合气体中CH3OH的体积百分含量与温度、 压强之间的关系如图1所示,图2表示在一定温度下,H2的平衡转化率与反应开始时两种反应物的投料物质的量之比(用X表示)、压强之间的关系。
CH3OH(g)。测得平衡时混合气体中CH3OH的体积百分含量与温度、 压强之间的关系如图1所示,图2表示在一定温度下,H2的平衡转化率与反应开始时两种反应物的投料物质的量之比(用X表示)、压强之间的关系。
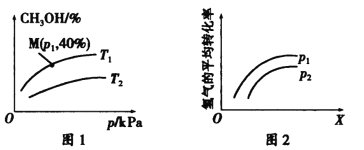
①压强相同时,温度为T1、T2时,反应达到平衡所需要的时间分别为t1、t2,则二者之间的相对大小为t1___ t2(填“>”、“<”、“=”或“无法确定”)。
②P1_____P2(填“>”、“<”、“=”或“无法确定”)。
③若a =2,b=4,则压强为P1、温度为T1时该反应的平衡常数K=______________。
④若在压强为P1、温度为T1时,向Q容器中同时加入等物质的量的CO、H2、CH3OH三种气体,则反应开始时,v(CH3OH)正_____v(CH3OH)逆(填“>”、“<”、“=”或“无法确定”)。
