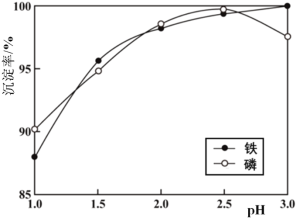
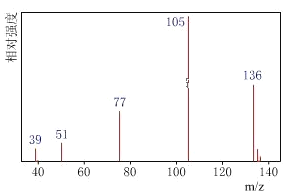
��Ŀ����
����Ŀ��ij�л�������A�����ϣ��������к�̼Ϊ70.59%������Ϊ 5.88%�����ຬ�������������з����ⶨ���л����������Է��������ͷ��ӽṹ��
����һ��������������֪A��������ͼ��
���������˴Ź����Dz��A�ĺ˴Ź���������4���壬�����֮��Ϊ1��2��2��3��
�����������ú�������Dz��A���ӵĺ�����ף���ͼ��

��1�������й���____�ֻ�ѧ������ͬ����ԭ�ӡ�
��2��A�ķ���ʽΪ____��
��3��������������һ���л���____��
��4��A�ķ�����ֻ��һ������������____(�����)��
a A����Է������� b A�ķ���ʽ
c A�ĺ˴Ź�������ͼ d A���ӵĺ������ͼ
��5��A�Ľṹ��ʽΪ________________________________��
���𰸡�4 C8H8O2 ���� bc 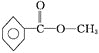
��������
��1���˴Ź�������֪A��4���壻
��2��N��C����N��H����N��O��=![]() ��
��![]() =4:4:1��A����Է�������Ϊ136��
=4:4:1��A����Է�������Ϊ136��
��3���Ӻ������֪��
��4����A�ĺ˴Ź���������4���壬�����֮��Ϊ1��2��2��3��A�ķ���ʽΪC8H8O2��
��5����������еõ����ӵ���Է���������������˴Ź���ͼ��֪�л����������Ļ�������������������ȷ���������������ţ���������ɵ�A�Ľṹ��ʽ��
��1���ɺ˴Ź�������֪A��4���壬����A�����й���4�ֻ�ѧ������ͬ����ԭ�ӣ�
��2����֪A�к�̼Ϊ70.59��������Ϊ 5.88��������23.53������ʽ����N��C����N��H����N��O��=![]() ��
��![]() =4:4:1�����A��ʵ��ʽΪC4H4O����A�ķ���ʽΪ��C4H4O��n ������A����Է�������Ϊ136���ɵ�n=2������ʽΪC8H8O2��
=4:4:1�����A��ʵ��ʽΪC4H4O����A�ķ���ʽΪ��C4H4O��n ������A����Է�������Ϊ136���ɵ�n=2������ʽΪC8H8O2��
��3���Ӻ������֪8��̼ԭ������6��C�ڱ����У�����C=O��C��O��C��C��H��Щ���ţ����Ʋ⺬�еĹ�����Ϊ����������A�����ࣻ
��4����A�ĺ˴Ź���������4���壬�����֮��Ϊ1��2��2��3��A�ķ���ʽΪC8H8O2����֪A������ֻ��һ��������Ϊ���ϵ���ԭ����3������ѡbc��
��5���������Ϸ�����֪��A�Ľṹ��ʽΪ�� ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ���±���Ԫ�����ڱ���һ����,�����Ԫ����-���ڱ��е�λ��,�ش��������⡣
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | �� | �� |
(1)����ԭ�ӽṹʾ��ͼ��___________��
(2)��ѧ��������õ�Ԫ����___________(��Ԫ�ط���)��
(3)������ԭ���У�ԭ�Ӱ뾶�ϴ����___________(��Ԫ�ط���)��
(4)�������뵼����ϵ���___________(������)��
(5)������������������Ӧ��ˮ���������Խ�ǿ����_________(�ѧʽ)��
(6)��ɫ��Ӧ�Ի�ɫ�����������Ľ���Ԫ����___________(��Ԫ�ط���)��
(7)Ԫ������������Ӧ��ˮ�����У������Ե���___________(�ѧʽ)��
(8)�ṹ��ʽΪ![]() ���л������ʽ��_____������̼Ԫ������Ԫ�ص�������m(C)��(H)=________��
���л������ʽ��_____������̼Ԫ������Ԫ�ص�������m(C)��(H)=________��