��Ŀ����
����Ŀ����Ҫ��ش��������⡣
(1)Al2(SO4)3��Һ�����Ե����ӷ���ʽ��____________________________��
(2)CuSO4��Һ�������ˮ��Ӧ�����ӷ���ʽ��____________________________��
(3)Mg-Al-NaOH��Һ��ɵ�ԭ��أ������ĵ缫��Ӧʽ��_________________________��
(4)CH3OH-O2ȼ�ϵ�أ�KOH��Һ������ʣ������ĵ缫��Ӧʽ��____________________��
(5)���Ե缫���CuSO4��Һ���ܷ�Ӧ�Ļ�ѧ����ʽ��______________________________��
(6)Na2C2O4��Һ�������غ㣺______________________________��
(7)Fe3+�Ļ�̬�����Ų�ʽ��______________________________��
(8)N2H4�Ľṹʽ��______________________________��
���𰸡�Al3++3H2OAl(OH)3+3H+ Cu2++4NH3H2O=[Cu(NH3)4]2++4H2O Al-3e-+4OH-�TAlO2-+2H2O CH3OH+8OH--6e-=CO32-+6H2O 2CuSO4+2H2O![]() 2Cu+2H2SO4+O2�� c(Na+)=2[c(C2O42-)+c(HC2O4-)+c(H2C2O4)] [Ar]3d5
2Cu+2H2SO4+O2�� c(Na+)=2[c(C2O42-)+c(HC2O4-)+c(H2C2O4)] [Ar]3d5 ![]()
��������
(1)Al2(SO4)3��Һ�д��������ӵ�ˮ�⣬������Һ�����ԣ��ʴ�Ϊ��Al3++3H2OAl(OH)3+3H+��
(2)CuSO4��Һ�������ˮ��Ӧ������ͭ�������ӣ��ʴ�Ϊ��Cu2++4NH3H2O=[Cu(NH3)4]2++4H2O��
(3)Mg-Al-NaOH��Һ��ɵ�ԭ��أ��ܷ�ӦΪAl������������Һ��Ӧ����ƫ�����ƺ������ķ�Ӧ��Alʧ���ӱ��������������ʴ�Ϊ��Al-3e-+4OH-�TAlO2-+2H2O��
(4)CH3OH-O2ȼ�ϵ�أ�KOH��Һ������ʣ������״�ʧȥ�������ɶ�����̼�����ڵ����Ϊ����������Һ�����Է�Ӧ������̼������ӣ������ĸ����缫��ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O��
(5)�������ͭ��Һ����ͭ���ʡ����������ᣬ�ʴ�Ϊ��2CuSO4+2H2O![]() 2Cu+2H2SO4+O2����
2Cu+2H2SO4+O2����
(6)Na2C2O4��Һ�������ӵ�Ũ�ȵ��ں�̼ԭ������Ũ��֮�͵Ķ������ʴ�Ϊ��c(Na+)=2[c(C2O42-)+c(HC2O4-)+c(H2C2O4)]����
(7)FeԪ��Ϊ26��Ԫ�أ�ʧȥ�����������������Fe3+���ʻ�̬�����Ų�ʽΪ��[Ar]3d5��
(8) N2H4�ǹ��ۻ������ԭ�Ӻ���ԭ���γɹ��ۼ�����ԭ�Ӻ͵�ԭ�Ӽ�Ҳ�γɹ��ۼ����ṹʽΪ��![]() ��
��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��Mn(H2PO4)2��2H2O��һ�ְ�ɫ���壬������ˮ�������ڻ�е�豸���������������̿�(��Ҫ�ɷ�ΪMnO2��������������Fe2O3��FeO��Al2O3)Ϊԭ���Ʊ�Mn(H2PO4)2��2H2O��������ͼ��
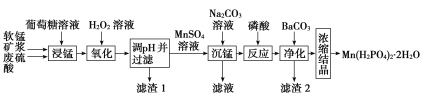
��1�����̿�Ҫ���Ƴɿ�Ŀ����__��������(C6H12O6)��MnO2��Ӧʱ������ΪMnSO4��CO2��H2O���÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ__��
��2����H2O2��Һ��������ʱ������Ӧ�����ӷ���ʽΪ��__��
��3����֪���ֽ������ӵ��������↑ʼ��������ȫ������pH���������pH��������ʱ��Ӧ������pH��ΧΪ__������1����Ҫ�ɷ�Ϊ__(�ѧʽ)��
�������� | ��ʼ������pH | ��ȫ������pH |
Fe3+ | 1.8 | 3.2 |
Al3+ | 3.0 | 5.0 |
Fe2+ | 5.8 | 8.8 |
Mn2+ | 7.8 | 9.8 |
��4���������������Ӧ�Ļ�ѧ����ʽΪ__��
��5��ij���������������Ʊ�Mn(H2PO4)2��2H2O����֪���̿���MnO2�ĺ���Ϊ87%��������������Ԫ�ص������Ϊ9%����1�ָ����̿���Ƶ�Mn(H2PO4)2��2H2O__t��