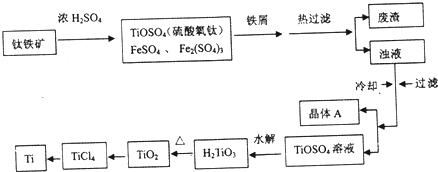
��Ŀ����
10����NH4��2SO4�dz����Ļ��ʺͻ���ԭ�ϣ������ֽ⣮ij��ȤС����̽����ֽ���[��������]��NH4��2SO4��260���400��ʱ�ֽ���ﲻͬ��
[ʵ��̽��]��С����ѡ����ͼ��ʾװ�ý���ʵ�飨�гֺͼ���װ���ԣ�
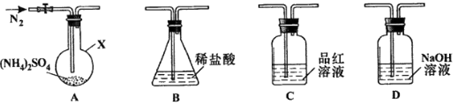
ʵ��1������װ��A-B-C-D����������ԣ���ͼʾ�����Լ���װ��Bʢ0.5000mol/L����70.00mL����ͨ��N2�ž���������260�����װ��Aһ��ʱ�䣬ֹͣ���ȣ���ȴ��ֹͣͨ��N2��Ʒ����Һ����ɫ��ȡ��װ��B������ָʾ������0.2000mol/L NaOH��Һ�ζ�ʣ�����ᣬ�յ�ʱ����NaOH��Һ25.00mL��������ζ������Һ����SO42-��
��1������X��������Բ����ƿ��
��2���ζ�ǰ�����в�������ȷ˳����dbaec������ĸ��ţ���
a��ʢװ0.2000mol/L NaOH��Һ b����0.2000mol/L NaOH��Һ��ϴ
c����������¼ d����©����ϴ e���ž��ζ��ܼ�������ݲ�����Һ��
��3��װ��B����Һ������������ʵ�����0.03mol
ʵ��2������װ��A-D-B����������ԣ���ͼʾ���¼����Լ���ͨ��N2�ž���������400�����װ��A����NH4��2SO4��ȫ�ֽ������ֹͣ���ȣ���ȴ��ֹͣͨ��N2���۲쵽װ��A��D֮��ĵ���������������ɫ���壮�����飬�ð�ɫ�����װ��D����Һ����SO32-����SO42-����һ���о����֣�����������������
��4������װ��D����Һ����SO32-����SO42-��ʵ�������������ȡ����D��Һ���Թ��У���������BaCl2��Һ���а�ɫ�������ɣ��ټ������ᣬ��ɫ������ȫ�ܽ⣬���ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
��5��װ��B����Һ���յ�������NH3��
��6����NH4��2SO4��400��ֽ�Ļ�ѧ����ʽ��3��NH4��2SO4$\frac{\underline{\;400��\;}}{\;}$4NH3��+3SO2��+6H2O��+N2����
���� ʵ��1����1������XΪԲ����ƿ��
��2���ζ�ǰ���ȼ��ζ����Ƿ�©ˮ���ٽ�����ϴ��Ȼ���ñ�Һ��ϴ����ע���Һ���ž��ζ���������ݲ�����Һ�棬��������¼���ζ�ǰ����ɣ�
��3�����������������Ƽ���Bװ����ʣ���HCl���μӷ�Ӧ��HCl���շֽ����ɵ�NH3��������Ӧ��NH3+HCl=
NH4Cl��������������NH3�����ʵ�����
ʵ��2����4��ȡD��Һ���Թ��У���������BaCl2��Һ���ټ������ᣬ��ɫ������ȫ�ܽ������ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
��5��װ��D����Һ����SO32-��˵���ֽ�����SO2��װ��A��D֮��ĵ���������������ɫ���壬��ɫ����Ӧ�Ƕ�����������ˮ�γɵ��Σ�װ��B����Һ���յ������ǰ�����
��6���ɣ�5���з�����֪����NH4��2SO4��400��ֽ�ʱ����NH3��SO2��H2O���ɣ�SԪ�ػ��ϼ۽��ͣ����ݵ���ת���غ㣬ֻ��ΪNԪ�ػ��ϼ����ߣ�����������������˵������N2����ƽ��д����ʽ��
��� �⣺��1��������X�Ľṹ��֪��XΪԲ����ƿ���ʴ�Ϊ��Բ����ƿ��
��2���ζ�ǰ���ȼ��ζ����Ƿ�©ˮ���ٽ�����ϴ��Ȼ���ñ�Һ��ϴ����ע���Һ���ž��ζ���������ݲ�����Һ�棬��������¼���ζ�ǰ����ɣ�����ȷ��˳��Ϊ��dbaec��
�ʴ�Ϊ��dbaec��
��3���ζ�ʣ�����ᣬ�յ�ʱ����NaOHΪ0.025L��0.2mol/L=0.005mol����ʣ��HClΪ0.005mol����μӷ�Ӧ��HClΪ0.07L��0.5mol/L-0.005mol=0.03mol���μӷ�Ӧ��HCl���շֽ����ɵ�NH3��������Ӧ��NH3+HCl=NH4Cl��������NH3�����ʵ���Ϊ0.03mol��
�ʴ�Ϊ��0.03��
��4�����װ��D����Һ����SO32-����SO42-��ʵ������������ǣ�ȡ����D��Һ���Թ��У���������BaCl2��Һ���а�ɫ�������ɣ��ټ������ᣬ��ɫ������ȫ�ܽ⣬���ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
�ʴ�Ϊ��ȡ����D��Һ���Թ��У���������BaCl2��Һ���а�ɫ�������ɣ��ټ������ᣬ��ɫ������ȫ�ܽ⣬���ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
��5��װ��D����Һ����SO32-��˵���ֽ�����SO2��װ��A��D֮��ĵ���������������ɫ���壬��ɫ����Ӧ�Ƕ�����������ˮ�γɵ��Σ�װ��B����Һ���յ������ǰ�����
�ʴ�Ϊ��NH3��
��6���ɣ�5���з�����֪����NH4��2SO4��400��ֽ�ʱ����NH3��SO2��H2O���ɣ�SԪ�ػ��ϼ۽��ͣ����ݵ���ת���غ㣬ֻ��ΪNԪ�ػ��ϼ����ߣ�����������������˵������N2���ֽⷴӦ����ʽΪ��3��NH4��2SO4$\frac{\underline{\;400��\;}}{\;}$4NH3��+3SO2��+6H2O��+N2����
�ʴ�Ϊ��3��NH4��2SO4$\frac{\underline{\;400��\;}}{\;}$4NH3��+3SO2��+6H2O��+N2����
���� ���⿼�黯ѧʵ�飬�漰��ѧ�������ζ�������ʵ�鷽����ơ���ѧ���㡢�����ƶϡ���ѧ����ʽ��д�ȣ��Ƕ�ѧ���ۺ������Ŀ��飬�ϺõĿ���ѧ����������������֪ʶǨ�����������Ѷ��еȣ�

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�| A�� | ��ˮ�����Ҵ�������CCl4 | |
| B�� | ��ȼ�շ�������顢��Ȳ��������̼ | |
| C�� | ��̼������Һ�����Ҵ���������������� | |
| D�� | �����Ը��������Һ������Ȳ����ϩ�ͱ��� |
| A�� | 1.0L1.0mo1•L-1��Na2SO4ˮ��Һ�к��е���ԭ����Ϊ4NA | |
| B�� | 25��ʱpH=13��NaOH��Һ�к���OHһ����ĿΪ0.1NA | |
| C�� | 2.3g�������������������Ӧ�������Ƿ����ת�Ƶ�������Ϊ0.1NA | |
| D�� | ��״���£�2.24LCl2����ˮ��ת�Ƶĵ�����ĿΪ0.1NA |
| A�� | �٢ܢ� | B�� | �ݢܢ� | C�� | �ڢ٢� | D�� | �ڢ٢� |
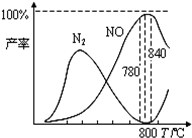 �������������Ṥҵ�Ļ�������ij����������ֻ��������
�������������Ṥҵ�Ļ�������ij����������ֻ��������������Ӧ��
�ڸ���Ӧ��
��4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H=-905kJ/mol
��4NH3��g��+3O2��g��?2N2��g��+6H2O��g����H=-1268kJ/mol
�й����ʲ������¶ȵĹ�ϵ��ͼ������˵����ȷ���ǣ�������
| A�� | ��ѹ�����NH3����NO��ת���� | |
| B�� | ��ҵ�ϰ����������� NOʱ����Ӧ�¶���ÿ�����780������ | |
| C�� | �ﵽƽ����������������䣬�ٳ���2 mol O2��Ӧ�ٵ�ƽ�ⳣ��K���ֲ��� | |
| D�� | N2����ΪNO���Ȼ�ѧ����ʽΪ��N2��g��+O2��g��?2NO��g����H=+363 kJ/mol |
| A�� | ��ͭ�ᴿʱ������ͭ��Һ�����Һ����ͭ������ | |
| B�� | ������ͭʱ����������������������Ӧ | |
| C�� | �ö��Ե缫���CuSO4��Һһ��ʱ����ټ��봿����CuO������ʹ��Һ�ָ���ԭ���ijɷֺ�Ũ�� | |
| D�� | ��ͭ�ᴿʱ����Fe��Zn��Ag�����ʻ�����ڵ��۵ײ��γ������� |
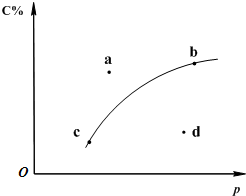 ���ڿ��淴Ӧ2A������+B��g��?2C��g������ͼ����������һ��ʱ��Ӧ��C�İٷֺ�����ѹǿ�Ĺ�ϵ���ߣ��ش��������⣺
���ڿ��淴Ӧ2A������+B��g��?2C��g������ͼ����������һ��ʱ��Ӧ��C�İٷֺ�����ѹǿ�Ĺ�ϵ���ߣ��ش��������⣺