��Ŀ����
��ÿ��2�֣���12�֣�ʵ����������220mL 0.2 mol��L��1��̼������Һ���ش��������⣺
�ɹ�ѡ�������������Ͳ�ڽ�ͷ�ιܢ�������ƽ��ҩ�ע���ƿ���ձ�
��1��ͨ�������֪��Ӧ��������ƽ��ȡ g̼���ƾ��壨Na2CO3?10H2O��
��2�����ƹ�����Ҫ������______________������ţ�����ȱ�ٵ�������
��3������ʱ����ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ�_____________��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1~2cm��
��4�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족������û�н���A����_____________��������ʱ���ӿ̶���______________��
�ɹ�ѡ�������������Ͳ�ڽ�ͷ�ιܢ�������ƽ��ҩ�ע���ƿ���ձ�
��1��ͨ�������֪��Ӧ��������ƽ��ȡ g̼���ƾ��壨Na2CO3?10H2O��
��2�����ƹ�����Ҫ������______________������ţ�����ȱ�ٵ�������
��3������ʱ����ȷ�IJ���˳���ǣ�����ĸ��ʾ��ÿ����ĸֻ����һ�Σ�_____________��
| A����30mLˮϴ���ձ�2~3�Σ�ϴ��Һ��ע������ƿ |
| B����������ƽȷ��ȡ�����Na2CO3����������������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ� |
| C��������ȴ����Һ�ز�����ע��250mL������ƿ�� |
| D��������ƿ�ǽ�����ҡ�� |
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1~2cm��
��4�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족������û�н���A����_____________��������ʱ���ӿ̶���______________��
��ÿ��2�֣���12�֣�(1) 14.3 (2) �ڢۢܢޣ� 250ml����ƿ���������� (3) BCAFED (4)ƫ�� ƫ��
����һ�����ʵ���Ũ����Һ������
��1������û��220ml��������ƿ������Ӧ������250ml�ģ������ʵ����ʵ�����0.25L��0.2mol/L��0.05mol��������ҪNa2CO3?10H2O��������0.05mol��286g/mol��14.3g��
��2���������ƹ��̿��жϣ���Ҫ�������Ǣڢۢܢޡ���ȱ��250ml����ƿ����������
��3���������ƹ��̿�֪����ȷ�IJ���˳����BCAFED��
��4�����û��ϴ�ӣ������ʵ����ʵ���ƫС���ⶨ���ƫ�ͣ�����ʱ���ӿ̶��ߣ�������ƿ����Һ�����ƫС���ⶨ���ƫ�ߡ�
��1������û��220ml��������ƿ������Ӧ������250ml�ģ������ʵ����ʵ�����0.25L��0.2mol/L��0.05mol��������ҪNa2CO3?10H2O��������0.05mol��286g/mol��14.3g��
��2���������ƹ��̿��жϣ���Ҫ�������Ǣڢۢܢޡ���ȱ��250ml����ƿ����������
��3���������ƹ��̿�֪����ȷ�IJ���˳����BCAFED��
��4�����û��ϴ�ӣ������ʵ����ʵ���ƫС���ⶨ���ƫ�ͣ�����ʱ���ӿ̶��ߣ�������ƿ����Һ�����ƫС���ⶨ���ƫ�ߡ�

��ϰ��ϵ�д�
 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д� ����һ������ܼƻ�ϵ�д�
����һ������ܼƻ�ϵ�д�
�����Ŀ
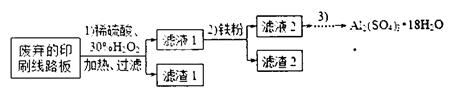
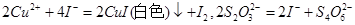
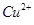 �������� ���ƫ�ߡ�����ƫ
�������� ���ƫ�ߡ�����ƫ
 ��ȥ���±��г��˼������������������������pH����ʼ������pH����������Ũ��Ϊ1��0mo1��L-1���㣩��
��ȥ���±��г��˼������������������������pH����ʼ������pH����������Ũ��Ϊ1��0mo1��L-1���㣩��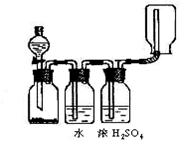
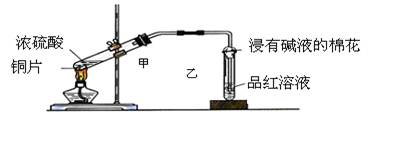
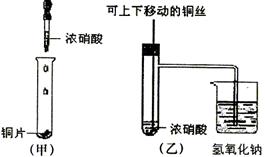
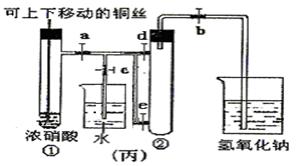
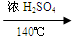 C2H4 ��+ H2O
C2H4 ��+ H2O