��Ŀ����
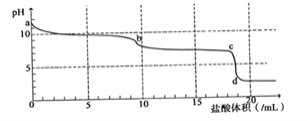
����Ŀ��25��ʱ����Ũ�Ⱦ�Ϊ0.1 mol/L������ֱ�ΪVa ��Vb ��HA��Һ��BOH��Һ����ͬ����Ȼ�ϣ�����Va + Vb = 100 mL�� Va��Vb����Һ��pH�Ĺ�ϵ��ͼ��ʾ������˵������ȷ����

A. Ka(HA)=Kb(BOH)=10-5
B. b��ʱ�� c(B+)=c(A-)��c(H+)=c(OH-)
C. c��ʱ�� 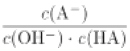 ���¶����߲���
���¶����߲���
D. a��c������ˮ�ĵ���̶���������С.
���𰸡�C
����������ͼ����Ϣ��֪��Va = 100 mLʱ��pH=3��c(H+)=![]() ����HAΪ���Vb = 100 mLʱ��pH=11��c(OH-)=
����HAΪ���Vb = 100 mLʱ��pH=11��c(OH-)=![]() ����BOHΪ���Ka(HA)=Kb(BOH)=
����BOHΪ���Ka(HA)=Kb(BOH)=![]() 10-5������ͬ�����£����ߵĵ���̶���ͬ��
10-5������ͬ�����£����ߵĵ���̶���ͬ��![]() ΪA����ˮ�ⳣ���ĵ�����
ΪA����ˮ�ⳣ���ĵ�����
A. Ka(HA)=Kb(BOH)=![]() 10-5��A��ȷ��
10-5��A��ȷ��
B. b��ʱ����ͼ�ǡ����ȫ��Ӧ����BA��Һ��B+��A-��ˮ����ˮ��̶���ͬ��pH=7����Һ�����ԣ���c(B+)=c(A-)��c(H+)=c(OH-)��B��ȷ��
C. ![]() ΪA����ˮ�ⳣ���ĵ������¶����ߣ�ˮ�ⳣ���������c��ʱ��
ΪA����ˮ�ⳣ���ĵ������¶����ߣ�ˮ�ⳣ���������c��ʱ��![]() ���¶����߶���С��C����ȷ��
���¶����߶���С��C����ȷ��
D.��������ˮ�ĵ��룬���ε�ˮ��ٽ�ˮ�ĵ��룬���ԣ� a��c������ˮ�ĵ���̶���������С.��D��ȷ��
����ѡC��

��ϰ��ϵ�д�
�����Ŀ