��Ŀ����
����Ŀ���Ķ������й���Դ�IJ��ϣ��ش��й����⣺
��1���ڳ��£�1.01��105Paʱ��48g �״��������������г��ȼ�����ɶ�����̼��Һ̬ˮ���ų�1089kJ�����������ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ____________________________��
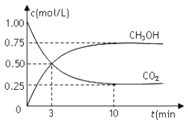
��2���������������о���������ѧ������ܵ��ת��������ͼ�ס�����װ���У����и����缫��ӦʽΪ____________________________�����е��������� _______ ���ƶ������������������������������缫��____�������������缫��ӦʽΪ____________________________
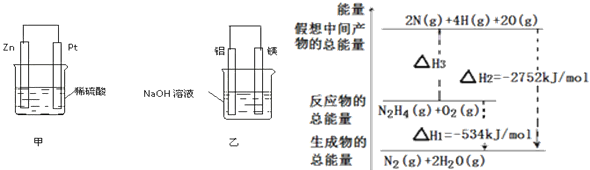
��3����(N2H4)����Ϊ�����������ȼ�ϣ��й��»�ѧ��Ӧ�������仯��ͼ��ʾ����֪����1mol��ѧ�����������(kJ)��N��NΪ944��O=OΪ500��N-NΪ154����Ӧ��ת��Ϊ�м�������H3=_________������1 mol N��H�������������__________ kJ��
��4����ҵ��������һ����Ҫ��Ӧ�ǣ�CO(g)+ H2O(g) =CO2(g) + H2(g),
��֪25��ʱ�� C(ʯī)+O2(g) = CO2(g) �� ��H1 = -394 kJmol-1
C(ʯī)+![]() O2(g) = CO(g)�� ��H2 = -111 kJmol-1
O2(g) = CO(g)�� ��H2 = -111 kJmol-1
H2(g)+ ![]() O2 (g)=H2O(g)�� ��H3= -242kJmol��1
O2 (g)=H2O(g)�� ��H3= -242kJmol��1
�Լ���25��ʱCO(g)+H2O(g)=CO2(g)+H2(g)�ķ�Ӧ�ȡ�H��____________kJmol-1��
���𰸡�CH3OH��l��+![]() O2(g)=CO2(g)+2H2O(l) ��H=-726kJ/moL Zn-2e-=Zn2+ �� �� 2H2O+2e-=H2��+2OH- +2218kJ/moL 391 -39kJ/moL
O2(g)=CO2(g)+2H2O(l) ��H=-726kJ/moL Zn-2e-=Zn2+ �� �� 2H2O+2e-=H2��+2OH- +2218kJ/moL 391 -39kJ/moL
��������
���ݸ�˹���ɣ�����֪�Ȼ�ѧ�������ʵ���ϵ�����мӼ�����Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ��ϵ����������Ӧ�ļӼ����ݴ˼��㡣
��1��48g CH3OH��������ȼ������CO2��Һ̬ˮ���ų�1089kJ������32g��1mol CH3OH��������ȼ������CO2��Һ̬ˮ���ų�726kJ���������ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽΪ��CH3OH(g)+![]() O2(g)�TCO2(g)+2H2O(l) ��H= -726kJ/mol��
O2(g)�TCO2(g)+2H2O(l) ��H= -726kJ/mol��
��2��ԭ����нϻ��õĽ����Ǹ�����ʧȥ���ӣ�����������Ӧ�����и����缫��ӦʽΪ��Zn-2e-=Zn2+��ԭ������������������ƶ�������Pt�缫�ƶ���
����þ�Ľ�����ǿ�����ģ���þ��������������Һ��Ӧ����������������Һ��Ӧ����ʧ������Ϊ����������������ˮ��������������ӵõ��Ӳ����������缫��ӦʽΪ2H2O+2e-=H2��+2OH-��
��3������ͼ���֪��N2H4(g)+O2(g)=2N(g)+4H(g)+2O(g)
��H3=2752kJ/mol-534kJ/mol=+2218kJ/mol��
��ѧ��Ӧ�У��ɼ��������������������1molN-H�����������Ϊx����ɼ��������յ�����=154+4x+500=2218����ã�x=391��
��4����֪��25��ʱ��
��C(ʯī)+O2(g)�TCO2(g) ��H1= -394kJ/mol
��C(ʯī)+![]() O2(g)�TCO(g) ��H2= -111kJ/mol
O2(g)�TCO(g) ��H2= -111kJ/mol
��H2(g)+![]() O2(g)�TH2O(g) ��H3= -242kJ/mol
O2(g)�TH2O(g) ��H3= -242kJ/mol
�ɸ�˹���ɣ���-��-�ڵã�CO(g)+H2O(g)�TCO2(g)+H2(g)������H=��H1-��H3-��H2= -111kJ/mol -(-394kJ/mol)-(-242kJ/mol)= -39kJ/mol��

 �Ƹ�С״Ԫ�������������ϵ�д�
�Ƹ�С״Ԫ�������������ϵ�д�����Ŀ�������Ƕ���������ͬ���칹�壬��ṹ��ʽ���е㼰�۵����±���ʾ��
�춡�� | �嶡�� | |
�ṹ��ʽ |
|
|
�е�/�� | 108 | 82.3 |
�۵�/�� | -108 | 25.5 |
����˵������ȷ����
A. ��ϵͳ���������춡������Ϊ��2-��-1-����
B. �춡���ĺ˴Ź�������������壬�����֮����1��2��6
C. ������ķ����ɽ��嶡���Ӷ��ߵĻ�����з������
D. ���ִ�������ȥ��Ӧ��õ�ͬһ��ϩ��
����Ŀ����һ�������»�ѧ��Ӧ��![]() �����ݻ���ͬ�ҹ̶�����ļס��ҡ������������������������·ֱ���������ͷ�Ӧ�ų�������
�����ݻ���ͬ�ҹ̶�����ļס��ҡ������������������������·ֱ���������ͷ�Ӧ�ų�������![]() ������У�
�������
���� |
|
| N2(mol) |
|
�� | 2 | 1 | 0 |
|
�� | 1 |
| 0 |
|
�� | 1 |
| 1 |
|
�����������ݣ�������������ȷ����
A.![]()
B.![]()
C.![]()
D.�����������·�Ӧ����![]() �������
�������![]()