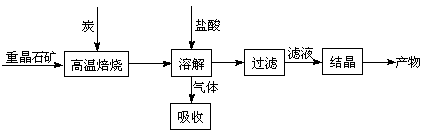
��Ŀ����
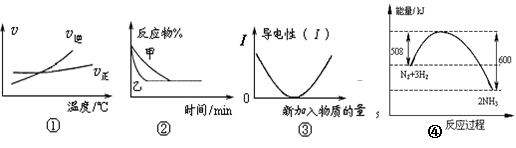
��10�֣��£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г������������Իش���������
��1����ȼ�ϵ��ԭ����ͼ��ʾ����ߵ缫�Ϸ����ĵ缫��ӦʽΪ_________��
��2���������N2O4��������������ȼ�ϣ���֪��

N2(g)��2O2(g)��2NO2(g) ��H ����67.7kJ��mol-1
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H ����534. 0kJ��mol-1
2NO2(g) N2O4(g) ��H ����52.7kJ��mol-1
N2O4(g) ��H ����52.7kJ��mol-1
��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ��________��
��3�������Ĺ�ҵ�������ð��ʹ�������Ϊԭ�ϻ�ã�д����Ӧ�����ӷ���ʽΪ_ __��
��4����ͼ��ʾ��A�������Ȳ����Ƴɵ����������ܱ�������B��һ�ͻ�ѧ��ʴ�����ڴ��ȵ�����ɱ�������ң�����ı���������嵯���ı���Բ��ƣ����ر�K2������1 mol NO2ͨ��K1��K3�ֱ����A��B�У���Ӧ��ʼʱA��B�������ͬ��Ϊa L��
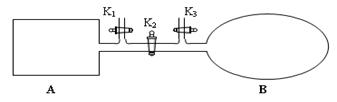
��B�п�ͨ��________________�жϿ��淴Ӧ2NO2 N2O4�Ѿ��ﵽƽ�⡣
N2O4�Ѿ��ﵽƽ�⡣
������K2��ƽ���B�������������0.4a L�����K2֮ǰ������B���Ϊ______L��
��������A�е���ƽ������ʱ��t s���ﵽƽ���������ѹǿΪ��ʼѹǿ��0.8������ ƽ����ѧ��Ӧ����v(NO2)����____________________(�ú�a��t�Ĵ���ʽ��ʾ)��
����ƽ�����A�������ٳ���0.5mol NO2�������µ���ƽ���ƽ��������NO2���������_______________���� �������С�����䡱����
��1����ȼ�ϵ��ԭ����ͼ��ʾ����ߵ缫�Ϸ����ĵ缫��ӦʽΪ_________��
��2���������N2O4��������������ȼ�ϣ���֪��

N2(g)��2O2(g)��2NO2(g) ��H ����67.7kJ��mol-1
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H ����534. 0kJ��mol-1
2NO2(g)
 N2O4(g) ��H ����52.7kJ��mol-1
N2O4(g) ��H ����52.7kJ��mol-1��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ��________��
��3�������Ĺ�ҵ�������ð��ʹ�������Ϊԭ�ϻ�ã�д����Ӧ�����ӷ���ʽΪ_ __��
��4����ͼ��ʾ��A�������Ȳ����Ƴɵ����������ܱ�������B��һ�ͻ�ѧ��ʴ�����ڴ��ȵ�����ɱ�������ң�����ı���������嵯���ı���Բ��ƣ����ر�K2������1 mol NO2ͨ��K1��K3�ֱ����A��B�У���Ӧ��ʼʱA��B�������ͬ��Ϊa L��

��B�п�ͨ��________________�жϿ��淴Ӧ2NO2
 N2O4�Ѿ��ﵽƽ�⡣
N2O4�Ѿ��ﵽƽ�⡣������K2��ƽ���B�������������0.4a L�����K2֮ǰ������B���Ϊ______L��
��������A�е���ƽ������ʱ��t s���ﵽƽ���������ѹǿΪ��ʼѹǿ��0.8������ ƽ����ѧ��Ӧ����v(NO2)����____________________(�ú�a��t�Ĵ���ʽ��ʾ)��
����ƽ�����A�������ٳ���0.5mol NO2�������µ���ƽ���ƽ��������NO2���������_______________���� �������С�����䡱����
��10�֣���1��N2H4��4e����4OH����N2��4H2O
��2��2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g) ��H����947.6 kJ�� mol��1
(3) 2NH3+CIO-=N2H4+Cl-+H2O
��4��������B��������ټ�С��������ɫ���ٱ仯�����������𰸸��֣�
��0.7L����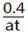 mol��L-1��s-1�ܱ�С
mol��L-1��s-1�ܱ�С
��2��2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g) ��H����947.6 kJ�� mol��1
(3) 2NH3+CIO-=N2H4+Cl-+H2O
��4��������B��������ټ�С��������ɫ���ٱ仯�����������𰸸��֣�
��0.7L����
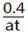 mol��L-1��s-1�ܱ�С
mol��L-1��s-1�ܱ�С��1��ԭ����и���ʧȥ���ӣ������õ����ӡ�������ٸ���ͨ�룬����������ͨ�롣����Ϊ��������������أ�������༴������Ӧʽ��N2H4��4e����4OH����N2��4H2O��
��2�����ݸ�˹���ɿ�֪���ڡ�2���٣��ۼ��õ���Ӧʽ2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g)�����Ը÷�Ӧ�ķ�Һ��H����534. 0kJ��mol-1��2��67.7kJ��mol-1��52.7kJ��mol-1����947.6 kJ�� mol��1��
��3�����ݷ�Ӧ����������֪���÷�Ӧ�����ӷ���ʽ��2NH3+CIO-=N2H4+Cl-+H2O��
��4���������ڷ�Ӧ���������������DZ仯�ģ����Կ���ͨ������B������仯���жϣ�����ΪNO2�Ǻ���ɫ�����壬����Ҳ����ͨ��������ɫ�ı仯���жϡ�������B��������ټ�С��������ɫ���ٱ仯ʱ����˵����Ӧ����ƽ��״̬��
�ڴ�K2�����൱�����ڵ��µ�ѹʱ��ƽ�⣬���ƽ��ʱ��Ч�ġ����ڴ�ʱ��Ӧ������ʵ�����B�еĶ��������Դ�K2֮ǰ������B���Ϊ��aL��0.4aL����2��0.7aL��
��������NO2�����ʵ�����x������ݷ���ʽ2NO2=N2O4��֪������N2O4�����ʵ�����x/2,��1��x��x/2��0.8�����x��0.4mol������NO2�ķ�Ӧ������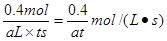 ��
��
�����ڷ�Ӧ��������ﶼ��һ�֣������ٳ���NO2�����൱��������ѹǿ��ƽ��������Ӧ�����ƶ������NO2�ĺ������͡�
��2�����ݸ�˹���ɿ�֪���ڡ�2���٣��ۼ��õ���Ӧʽ2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g)�����Ը÷�Ӧ�ķ�Һ��H����534. 0kJ��mol-1��2��67.7kJ��mol-1��52.7kJ��mol-1����947.6 kJ�� mol��1��
��3�����ݷ�Ӧ����������֪���÷�Ӧ�����ӷ���ʽ��2NH3+CIO-=N2H4+Cl-+H2O��
��4���������ڷ�Ӧ���������������DZ仯�ģ����Կ���ͨ������B������仯���жϣ�����ΪNO2�Ǻ���ɫ�����壬����Ҳ����ͨ��������ɫ�ı仯���жϡ�������B��������ټ�С��������ɫ���ٱ仯ʱ����˵����Ӧ����ƽ��״̬��
�ڴ�K2�����൱�����ڵ��µ�ѹʱ��ƽ�⣬���ƽ��ʱ��Ч�ġ����ڴ�ʱ��Ӧ������ʵ�����B�еĶ��������Դ�K2֮ǰ������B���Ϊ��aL��0.4aL����2��0.7aL��
��������NO2�����ʵ�����x������ݷ���ʽ2NO2=N2O4��֪������N2O4�����ʵ�����x/2,��1��x��x/2��0.8�����x��0.4mol������NO2�ķ�Ӧ������
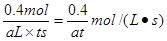 ��
�������ڷ�Ӧ��������ﶼ��һ�֣������ٳ���NO2�����൱��������ѹǿ��ƽ��������Ӧ�����ƶ������NO2�ĺ������͡�

��ϰ��ϵ�д�
�����Ŀ
 4CO(g) + BaS(s) ��H1 ����571.2 kJ��mol-1 ��
4CO(g) + BaS(s) ��H1 ����571.2 kJ��mol-1 �� �� ��[Ksp(AgBr)��5.4��10-13��Ksp(AgCl)��2.0��10-10]
�� ��[Ksp(AgBr)��5.4��10-13��Ksp(AgCl)��2.0��10-10] 2CH3OH(g) ��H= 37Kj��mol-1
2CH3OH(g) ��H= 37Kj��mol-1 CH3OH(g) ��H <0���ֽ�l0mol CO��20mol H2�����ܱ������У��ڴ��������·�����Ӧ���ɼ״���CO��ƽ��ת���ʣ�
CH3OH(g) ��H <0���ֽ�l0mol CO��20mol H2�����ܱ������У��ڴ��������·�����Ӧ���ɼ״���CO��ƽ��ת���ʣ�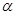 �����¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��
�����¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ��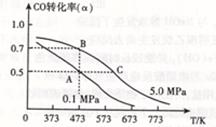
 2AB3��g���� �Ħ�H>0
2AB3��g���� �Ħ�H>0 H2��I2(g)
H2��I2(g) 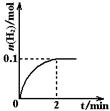
 H����OH����ƽ�� �ƶ�����������ҡ����ߡ�����������Ҫ��С����H2�����ʶ��ֲ�Ӱ�����H2��������Ӧ�������м��������Լ��е� ��
H����OH����ƽ�� �ƶ�����������ҡ����ߡ�����������Ҫ��С����H2�����ʶ��ֲ�Ӱ�����H2��������Ӧ�������м��������Լ��е� �� 7N2��12H2O�ɴ���NO2����ת��3.6mol����ʱ�����ɵ�N2�ڱ�״������ L��
7N2��12H2O�ɴ���NO2����ת��3.6mol����ʱ�����ɵ�N2�ڱ�״������ L�� CH3OH��g������ƽ����ø����Ũ�����£�
CH3OH��g������ƽ����ø����Ũ�����£�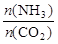 ��4ʱ��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ��
��4ʱ��CO2��ת������ʱ��ı仯��ϵ����ͼ��ʾ��