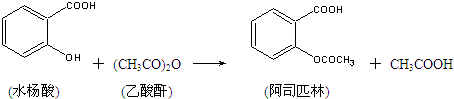
��Ŀ����
��10�֣���˾ƥ�֣�����ˮ���ᣬ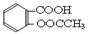 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128~135�档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128~135�档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�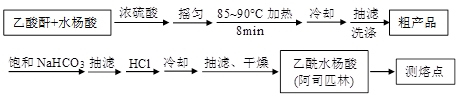
��Ҫ�Լ��Ͳ�Ʒ����������
| �� �� | ��Է������� | �۵��е㣨�棩 | ˮ |
| ˮ���� | 138 | 158(�۵�) | �� |
| ������ | 102 | 139.4(�е�) | ��Ӧ |
| ����ˮ���� | 180 | 135(�۵�) | �� |
���Ʊ���˾ƥ�ֵĻ�ѧ����ʽ ��
�ƺϳɰ�˹ƥ��ʱ����δ���ֽᾧ���ɲ�ȡ�Ĵ�ʩ��
_____________________________��
���ᴿ�ֲ����м��뱥��NaHCO3��Һ��û��CO2����Ϊֹ���ٳ��ˣ���ӱ���NaHCO3��Һ��Ŀ������������������������������������������������
����һ�ָĽ����ᴿ��������Ϊ�ؽᾧ�ᴿ�����������£�
 �Ľ����ᴿ�����м��Ȼ�����װ����ͼ��ʾ
�Ľ����ᴿ�����м��Ȼ�����װ����ͼ��ʾ
��a���������� ������ˮ������������ ���b����c�� ����
���ؽᾧ�ᴿ�����ò�Ʒ���л�����Ҫ��ԭ�����ٵ�ԭ����
_________________________________________________��
�ɼ����Ʒ���Ƿ���ˮ����Ļ�ѧ������ ��
�ʸ�ѧϰС����ʵ����ԭ��������2.76 gˮ���ᡢ7.5 mL������������1.08 g/cm3�������ճ�����Ʒm =" 2.92g" ������������ˮ����IJ���Ϊ ��
(1)
(2)�ò�����Ħ��ƿ�ڲ���װ�÷��ڱ�ˮԡ����ȴ��
(3)ʹ����ˮ������NaHCO3��Һ����ת��Ϊ������ˮ������ˮ�����ƣ����������ʾۺ�����롣
(4)��������ƿ��c
����Ϊˮ������������������������ȴ�ᾧʱ��ˮ�������ܽ������������к��ٽᾧ����
(5)ȡ�����ᾧ���Թ��У�������ˮ�ܽ⣬�μ�FeCl3��Һ��������ɫ��ˮ���ᡣ
(6)81.1%
���������������1���Ʊ�����ˮ����ķ�ӦΪ��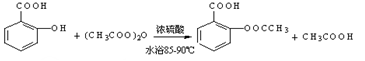
��2�����û�г��ֽᾧ˵����Һδ���ͻ��پ��ˣ�����ò�����Ħ��ƿ�ڲ���װ�÷��ڱ�ˮԡ����ȴ��
��3������ˮ������NaHCO3��Һ��Ӧ��������ˮ�����ƣ�����ˮ������������ˮ���������л������Կ������л�����з��롣
��4��a���������ƽ�������ƿ������ˮӦ�����½��ϳ�������ˮ�������л������������������У���˺��ѽᾧ������
��5��ˮ����������ˮ����IJ������ˮ�������з��ǻ�������ȡ�����ᾧ���Թ��У�������ˮ�ܽ⣬�μ�FeCl3��Һ��������ɫ��ˮ���ᡣ
��6��n(ˮ����)= ��n(������)=
��n(������)= ��������������������ˮ����0.02mol�������Ϊ
��������������������ˮ����0.02mol�������Ϊ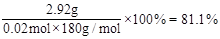 ��
��
���㣺�л���ķ����ᴿ
�����������ۺ���ǿ���ǽ�����߿����ȵ㣬Ӧ�����ӡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�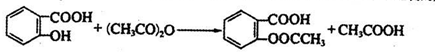
| A����˾ƥ�ַ�������1������̼ԭ�� | B��ˮ����Ͱ�˾ƥ�ֶ����ڷ����廯���� | C������FeCl3��Һ���鰢˾ƥ�����Ƿ���δ��Ӧ��ˮ���� | D��1mol��˾ƥ��������2mol��NaOH��Һ��Ӧ |
 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[��CH3CO��2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�