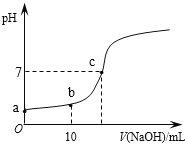
��Ŀ����
����Ŀ�����л�ѧ����ʽ��д��ȷ���ǣ� ��
A.�����������Ʊ���CH3COOH + CH3CH218OH![]() CH3COOCH2CH3 + H218O
CH3COOCH2CH3 + H218O
B.��ȩ������������ͭ����Һ���ȣ�CH3CHO + 2Cu(OH)2 + NaOH ![]() CH3COONa + Cu2O��+ 3H2O
CH3COONa + Cu2O��+ 3H2O
C.��������Һ��ͨ������������̼��2![]() + CO2 + H2O
+ CO2 + H2O ![]() 2
2![]() + Na2CO3
+ Na2CO3
D.�������缫��ⱥ���Ȼ�����Һ��2NaCl + 2H2O![]() 2NaOH + H2��+ Cl2��
2NaOH + H2��+ Cl2��
���𰸡�B
��������
A. �����������Ʊ����Ҵ������ᷢ��������Ӧ��������������ˮ����Ӧʱ����ǻ������⣬��Ӧ�Ļ�ѧ����ʽΪ��CH3COOH + CH3CH218OH![]() CH3CO18OCH2CH3 + H2O��ѡ��A����
CH3CO18OCH2CH3 + H2O��ѡ��A����
B. ��ȩ������������ͭ����Һ���ȣ���Ӧ���������ơ�������ͭ������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3CHO + 2Cu(OH)2 + NaOH ![]() CH3COONa + Cu2O��+ 3H2O��ѡ��B��ȷ��
CH3COONa + Cu2O��+ 3H2O��ѡ��B��ȷ��
C. ��������Һ��ͨ������������̼����Ӧ���ɱ��Ӻ�̼�����ƣ���Ӧ�Ļ�ѧ����ʽΪ��![]() + CO2 + H2O
+ CO2 + H2O ![]()
![]() + NaHCO3��ѡ��C����
+ NaHCO3��ѡ��C����
D. �������缫��ⱥ���Ȼ�����Һ����ʧ���Ӳ����������ӣ��缫�ܷ�ӦʽΪ��Fe + 2H2O![]() Fe(OH)2+ H2����ѡ��D����
Fe(OH)2+ H2����ѡ��D����
��ѡB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����в����������۶���ȷ��������Ե���
ѡ�� | ���� | ���� | ���� |
A |
|
| ԭ��Һ������ |
B | ����ZnS�� | ���ɺ�ɫ���� |
|
C | �� | ��Һ����� | ���� |
D | �����������ֲ�Ʒ�м������� | ������������ | ����������Ʒ�л����Ҵ� |
A.AB.BC.CD.D
����Ŀ������������(C9H10O2)����ˮ����ζ������������ˮ�㾫�����쾫�ͣ���������ʳƷ��ҵ�У�Ҳ�������л��ϳ��м��塢�ܼ��ȡ����Ʊ�����Ϊ��

��֪��
��ɫ��״̬ | �е�(��) | �ܶ�(g��cm��3) | |
������ | ��ɫ��Ƭ״���� | 249 | 1.265 9 |
���������� | ��ɫ����Һ�� | 212.6 | 1.05 |
�Ҵ� | ��ɫ����Һ�� | 78.3 | 0.789 3 |
������ | ��ɫ����Һ�� | 80.8 | 0.731 8 |
*��������100 ���Ѹ��������
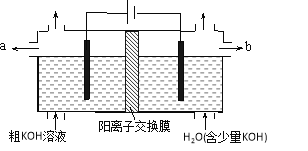
ʵ�鲽�����£�
a����100 mLԲ����ƿ�м���12.20 g�����ᡢ25 mL�Ҵ�(����)��20 mL �����飬�Լ�4 mLŨ���ᣬ��Ͼ��Ȳ������ʯ������ͼ��ʾװ�������������¶���65��70 ����Ȼ���2 h����Ӧʱ���������Ҵ���ˮ���γ�����������(�е�62.6 ��)��������������÷�ˮ�����Ϸ����ȥ��Ӧ���ɵ�ˮ��������������Ҵ���

b����Ӧ�������������ų���ˮ����Һ��ر��������������ȣ�����ˮ�����ռ�����Һ�岻���������ӣ�ֹͣ���ȡ�
c������ƿ�ڷ�ӦҺ����ʢ������ˮ���ձ��У����뱥��Na2CO3��Һ��
d���÷�Һ©���ֳ��л��㣬ˮ����25 mL������ȡ��Һ��Ȼ��ϲ����л��㡣�����Ȼ��ƣ��Դֲ�������������������Ѻ������£�����210��213 �����֡�
e������ϸ�ò�Ʒ���Ϊ12.86 mL��
�ش��������⣺
��1����Ӧ��Ũ����������ǣ�________________������a�м����ʯ���ã�____________���¶���65��70 ����ȵķ����ǣ�________________��
��2������A�������ǣ�________________���÷�Ӧ����Ϊ______________��
��3�����ڲ���d�еķ�Һ����������ȷ����________��
A��ˮ��Һ�м������ѣ�ת������Һ©���У����ϲ�����������Һ©����ת������������ҡ
B����ҡ���κ����Һ©���¿ڵIJ�����������
C����������ҡ���������ֳַ�Һ©�����ô�Һ��ֲ�
D���ų�Һ��ʱ���轫�������ϵİ��۶�©�����ϵ�С��
��4������ʵ���б���̼������Һ��������___________________��
��5�����ﵽ�÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���÷�Ӧ�Ѵﵽ��ѧƽ��״̬����(�����)_______________________��
�ٵ�λʱ�������1mol������������ͬʱ����1molˮ
�ڵ�λʱ�������1mol������������ͬʱ����1mol�Ҵ�
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol������
������Ӧ���������淴Ӧ���������
�ݺ����и����ʵ�Ũ�Ȳ��ٱ仯
������и����ʵ�Ũ�����