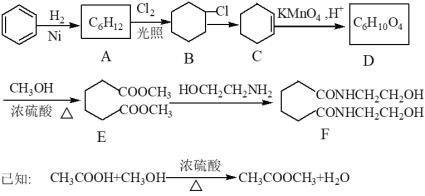
��Ŀ����
����Ŀ��Ũ����㷺Ӧ����ϡ�н�����ʪ��ұ��ƯȾ��ҵ�������ӹ�����ҩƷ���л�ҩ��������������С�
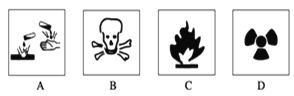
��.(1)��ʢ��Ũ������Լ�ƿ�ı�ǩ��Ӧӡ��ͼ��ʾ��־�е�____________��
(2)��12.0mol/LŨ��������230mL 0.3mol/L��ϡ���ᣬ��Ҫ����Ͳ��ȡŨ��������Ϊ______��
(3)��Һ���ƹ�������Ҫ�IJ������������ձ�������������Ͳ������____________________��
(4)��Һϡ�����������²�����
a����ȡŨ�����һ�������ˮ�����ձ���ϡ�ͣ�
b����������Ũ����������
c�����µߵ�ҡ�ȣ�
d��������ˮ���̶���1-2cm�ط������ý�ͷ�ιܼ�����ˮ����Һ����̶������У�
e����ϡ��Һת��������ƿ��ϴ���ձ��Ͳ�����������ϴ��Һת��������ƿ����
������ȷ�IJ���˳��Ϊ__________________________
(5)ʵ������е����²����ᵼ������������ҺŨ�ȣ�����ƫ��������ƫС��������������
a����ȡŨ����ʱ���ӣ�__________ ��
b����ȡŨ�������ϴ����Ͳ����ϴ��Һת��������ƿ��__________ ��
c��ʵ��ǰ������ƿ����������������ˮ��__________
���𰸡�A 6.3mL 250mL����ƿ����ͷ�ι� b��a��e��d��c ƫ�� ƫ�� ����
��������
��.(1)Ũ�����н�ǿ�ĸ�ʴ�ԣ�
(2) ����230mL 0.3mol/L��ϡ���ᣬӦѡ��250mL������ƿ������V1=![]() ���㣻
���㣻
(3)��Һ���ƹ�������Ҫ��Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ�
(4)��Һ���ƹ�������Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ�
(5) a����ȡŨ����ʱ���ӣ�����Ũ��������ƫС�����ʵ����ʵ���ƫС����Ũ��ƫ�ͣ�
b����ȡŨ�������ϴ����Ͳ����ϴ��Һת��������ƿ������Ũ��������ʵ���ƫ����Ũ��ƫ�ߣ�
c��ʵ��ǰ������ƿ����������������ˮ������Һ�е����ʼ��ܼ�����Ӱ�죬��Ũ�Ȳ��䡣
��.(1)Ũ�����н�ǿ�ĸ�ʴ�ԣ�Ӧ����A��ͼʾ��
(2) ����230mL 0.3mol/L��ϡ���ᣬӦѡ��250mL������ƿ����V1=![]() =
=![]() =6.25mL������Ͳ��ȡ6.3mL��
=6.25mL������Ͳ��ȡ6.3mL��
(3)��Һ���ƹ�������Ҫ��Ͳ���ձ�����������250mL����ƿ����ͷ�ιܣ�
(4)��Һ���ƹ�������Ϊ�����㡢��ȡ��ϡ�͡���ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȣ���˳��Ϊb��a��e��d��c��
(5) a����ȡŨ����ʱ���ӣ�����Ũ��������ƫС�����ʵ����ʵ���ƫС����Ũ��ƫ�ͣ�
b����ȡŨ�������ϴ����Ͳ����ϴ��Һת��������ƿ������Ũ��������ʵ���ƫ����Ũ��ƫ�ߣ�
c��ʵ��ǰ������ƿ����������������ˮ������Һ�е����ʼ��ܼ�����Ӱ�죬��Ũ�Ȳ��䡣

 ��ѧ����ϵ�д�
��ѧ����ϵ�д�����Ŀ���������ʵ����ѡ���װ�û�����(�г�װ������ȥ)��ȷ����
ѡ�� | A | B | C | D |
ʵ�� | ��CCl4��ȡ��ˮ�е�Br2 | ��ʳ��ˮ����ȡNaCl���� | ��KI��I2�Ĺ��������л���I2 | ����100 mL 0.100 0 mol��L-1 K2Cr2O7��Һ |
װ�û����� |
|
|
|
|
A. A B. B C. C D. D