��Ŀ����
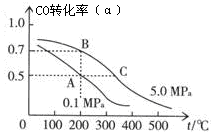
CO2�Ĺ̶��������ڽ������������ŷ��о�����Ҫ���ã���CO2����ϳɼ״�����������Ч�������ѹ�����������ۺ����õ�һ����;����CO2��H2�ڴ����������ܷ�����ӦCO2��3H2 CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
CH3OH��H2O����ü״������۲����뷴Ӧ�¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ����ش��������⣺
��1����״����ʵĴ�ʩ�� ��
��2������ͼ�����ݿ�֪����220 �桢5MPaʱ��CO2��ת����Ϊ ���ٽ��¶Ƚ�����
140�棬ѹǿ��С��2MPa����ѧ��Ӧ���ʽ� �������С �� ���䡰 ��ͬ����CO2��ת���ʽ� ��
��3��200��ʱ����0.100molCO2��0.275molH2����1L�ܱ������У��ڴ��������·�Ӧ�ﵽƽ�⡣��CO2��ת����Ϊ25%������¶��¸÷�Ӧ��ƽ�ⳣ��K�� ��(Ҫ��д����ʽ�ͼ�����)
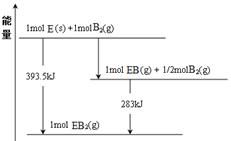
��4����֪��֪��CO��ȼ���ȡ�H����283.0KJ/mol��2H2(g)+O2(g)��2H2O(g) ��H����483.6KJ/mol��
CO(g)+2H2(g)��CH3OH(g) ��H����90.1KJ/mol��д��CO2��H2�ϳɼ״����Ȼ�ѧ����ʽ ��
��1�����¡���ѹ��2�֣� ��2��25%��2�֣� ��С��2�֣� ����2�֣�
��3��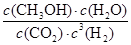 ��
��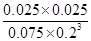 ��1.04 ��3�֣�
��1.04 ��3�֣�
��4��CO2(g)��3H2(g)===CH3OH(g)��H2O(g) ?H��-48.9 kJ/mol��3�֣�
���������������1������ͼ���֪�������¶ȵ����ߣ��״��IJ��ʽ��ͣ���˵������Ӧ�Ƿ��ȷ�Ӧ�����¶���ͬʱ������ѹǿ���״��IJ�������������״����ʵĴ�ʩ�ǽ��¡���ѹ��
��2������ͼ�����ݿ�֪����220 �桢5MPaʱ���״��IJ�����25%������̼ԭ���غ㣬1molCO2����1mol�״�����˼״��IJ��ʾ���CO2��ת���ʣ�����CO2��ת����Ϊ25%�����²�ͬʱ����ѹǿ����Ӧ���ʼ�С����������Ӧ�Ƿ��ȵ������С�Ŀ��淴Ӧ����˽��ºͽ���ѹǿƽ���������Ӧ�����ƶ�������CO2��ת��������
��3�� CO2(g)��3H2(g)===CH3OH(g)��H2O(g)
��ʼŨ�ȣ�mol/L��0.100 0.275 0 0
ת��Ũ�ȣ�mol/L��0.0250 0.0750 0.0250 0.0250
ƽ��Ũ�ȣ�mol/L��0.0750 0.200 0.0250 0.0250
���Ը��¶��·�Ӧ��ƽ�ⳣ��K��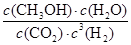 ��
��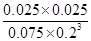 ��1.04
��1.04
��4��CO��ȼ���ȡ�H����283.0KJ/mol�������Ȼ�ѧ����ʽ��2CO(g)��O2(g)��2CO2(g) ��H����566.0KJ/mol������Ϊ��Ӧ��2H2(g)+O2(g)��2H2O(g) ��H����483.6KJ/mol����CO(g)+2H2(g)��CH3OH(g) ��H����90.1KJ/mol�����Ը��ݸ�˹���ɿ�֪��(�ۡ�2���٣���)��2�����õ���ӦCO2(g)��3H2(g)===CH3OH(g)��H2O(g)�����Ը÷�Ӧ�ķ�Ӧ��?H������90.1KJ/mol��2��566.0KJ/mol��483.6KJ/mol����2��-48.9 kJ/mol��
���㣺���鿼����������Է�Ӧ���ʺ�ƽ��״̬��Ӱ�죻��Ӧ���ʺ�ƽ�ⳣ���ļ����Լ���˹���ɵ�Ӧ��

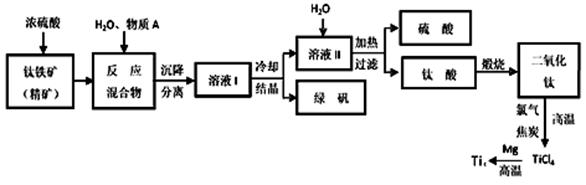
(14��)CO2��һ����Ҫ���������壬�о�CO2�����öԴٽ���̼���Ĺ���������Ҫ�����塣
��1�����ʯ��ʯīȼ�շ�Ӧ�е������仯��ͼ��ʾ��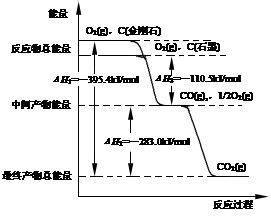
����ͨ��״���£����ʯ��ʯī�У� ������ʯ����ʯī�������ȶ���ʯī��ȼ����Ϊ kJ��mol��1��
��ʯī��CO2��Ӧ����CO���Ȼ�ѧ����ʽ�� ��
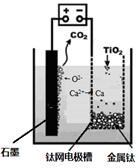
��2�����õ绯ѧ���ɽ�CO2ת��Ϊ���顣��д������������ˮ��Һ�������ʱ����ת���ĵ缫��Ӧ����ʽ ��
��3��CO2Ϊԭ�ϻ��ɺϳɶ������ʡ���ҵ�ϳ���CO2(g) ��H2(g)Ϊԭ�Ϻϳ��Ҵ���
����֪��H2O(l)=H2O(g) ��H=+44kJ��mol��1
CO(g)+H2O(g) CO2(g)+H2(g) ��H=��41.2kJ��mol��1
CO2(g)+H2(g) ��H=��41.2kJ��mol��1
2CO(g)+4H2 (g)  CH3CH2OH(g)+H2O(g) ��H= ��256.1kJ��mol��1��
CH3CH2OH(g)+H2O(g) ��H= ��256.1kJ��mol��1��
��2CO2(g)+6H2(g)  CH3CH2OH(g)+3H2O(l) ��H= ��
CH3CH2OH(g)+3H2O(l) ��H= ��
����ͼ��һ�����̵���Ϊԭ�Ϻϳ��Ҵ��Ĺ���ԭ��ʾ��ͼ��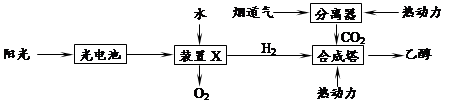
���������̵ķ���������˵����ȷ���� ��
| A�����������ٰ���4����ʽ������ת�� |
| B��װ��X��������ӦΪ��2H2O��4e��=4H++O2�� |
| C���ϳ����������Ҵ��ķ�Ӧ�ǻ��Ϸ�Ӧ |
| D�����������������ɫ��ѧ˼�� |
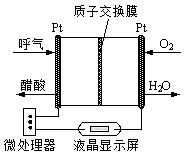
(1)���������ھ�������������ˮ������������ҵ�������ΪƯ����
�ٳ������������������ҿ������������е��ʷ�Ӧ���磺
6Ag(s)+O3(g)��3Ag2O(s)����H����235��8 kJ/mol��
��֪��2 Ag2O(s)��4Ag(s)+O2(g)����H��+62.2kJ/mol����O3ת��ΪO2���Ȼ�ѧ����ʽΪ ���ڿ�ѧ��P��Tatapudi��������ʹ�������������µ��ˮ�ķ����Ƶó�����������������Χ��ˮ�в������������������������ɹ������⣬�����缫��ӦʽΪ ��
| ʱ��/minŨ��(mol/L) | NO | N2 | CO2 |
| 0 | 1.00 | 0 | 0 |
| 10 | 0.58 | 0.21 | 0.21 |
| 20 | 0.40 | 0��30 | 0.30 |
| 30 | 0.40 | 0.30 | 0.30 |
| 40 | 0.32 | 0.34 | 0.17 |
| 50 | 0.32 | 0.34 | 0.l7 |
(2)�û���̿��ԭ��������������йط�ӦΪ�� C(s)+2NO(g)
 N2(g)+CO2(g)��ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2(g)+CO2(g)��ij�о�С����ij�ܱյ��������(��������������䣬��������������Բ���)�м���NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£���10 min��20 min����v(CO2)��ʾ�ķ�Ӧ����Ϊ
�ڸ��ݱ������ݣ�����T1��ʱ�÷�Ӧ��ƽ�ⳣ��K�� (������λС��)��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ��״̬���� (�������ĸ)��
A��������ѹǿ���ֲ���
B��2v��(NO)=v��(N2)
C��������CO2�������������
D�����������ܶȱ��ֲ���
��30 minʱ�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ��
��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת���� (����������䡱��С��)��
ͼa��1 mol NO2��1 mol CO��Ӧ����CO2��NO�����������仯ʾ��ͼ��ͼb�Ƿ�Ӧ�е�CO��NO��Ũ����ʱ��仯��ʾ��ͼ������ͼ��ش��������⣺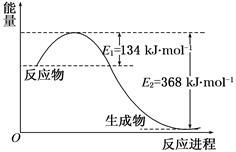
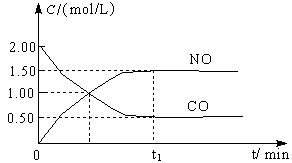
a b
(1)д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ ��
(2)�ӷ�Ӧ��ʼ��ƽ�⣬��NO2Ũ�ȱ仯��ʾƽ����Ӧ����v(NO2)�� ��
(3)���¶��¸÷�Ӧ��ƽ�ⳣ��K= ���¶Ƚ��ͣ�K ����������С�����䡱��
(4)�����¶Ⱥ��ݻ���ͬ�������ܱ������У�����ͬ��ʽͶ�뷴Ӧ���÷�Ӧ�ﵽƽ�ⅼ���й��������±���
| �� �� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1 mol NO2 1 mol CO | 2 mol NO 2 mol CO2 | 1 mol NO2��1 mol CO 1 mol NO��1 mol CO2 |
| ƽ��ʱc(NO) /mol��L-1 | 1.5 | 3 | m |
| �����仯 | �ų�a kJ | ����b kJ | �ų�c kJ |
| CO��NO��ת���� | ��1 | ��2 | ��3 |
��1+��2= �� a+b/2= ,m=
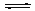 N2(g)+2CO2(g) ��H��0��
N2(g)+2CO2(g) ��H��0�� 
 CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��