��Ŀ����
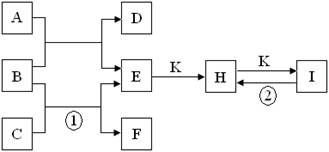
(10��)ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�����ͼ��ʾ�ı仯��

��֪B���������Zԭ�Ӹ�����C��������һ����
��ش��������⣺
(1) Ԫ��Xλ�� ���� ��
(2) Ԫ��Y��ԭ�ӽṹʾ��ͼ
(3) �õ���ʽ��ʾB���γɹ��̣�
(4) B��C���ȶ��Դ�С˳��Ϊ ���û�ѧʽ��ʾ��
(5) C��X��һ�����������ɻ�����A�Ļ�ѧ����ʽ

��֪B���������Zԭ�Ӹ�����C��������һ����
��ش��������⣺
(1) Ԫ��Xλ�� ���� ��
(2) Ԫ��Y��ԭ�ӽṹʾ��ͼ
(3) �õ���ʽ��ʾB���γɹ��̣�
(4) B��C���ȶ��Դ�С˳��Ϊ ���û�ѧʽ��ʾ��
(5) C��X��һ�����������ɻ�����A�Ļ�ѧ����ʽ
(1) �ڶ����ڢ�A�� ��2) 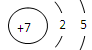
(3)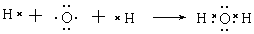
(4) H2O�� NH3 (5) 4NH3+5O2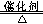 4NO+6H2O
4NO+6H2O
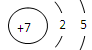
(3)
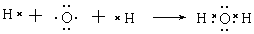
(4) H2O�� NH3 (5) 4NH3+5O2
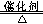 4NO+6H2O
4NO+6H2O���³�ѹ�¾�Ϊ��ɫ���嵥�ʵģ�һ���ǵ�������������������ΪB���������Zԭ�Ӹ�����C��������һ��������B��ˮ���ӣ�C�ǰ������ӣ���X��Y��Z�ֱ�������������������������A��B��C�ֱ���NO��H2O��NH3��ˮ���ɼ��Լ��γɵĹ��ۻ�����γɹ��̿�Ϊ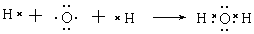 ���ǽ�����Խǿ���⻯��Խ�ȶ����ǽ�������O����N������ˮ���ȶ��Դ��ڰ����ġ�
���ǽ�����Խǿ���⻯��Խ�ȶ����ǽ�������O����N������ˮ���ȶ��Դ��ڰ����ġ�
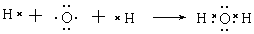 ���ǽ�����Խǿ���⻯��Խ�ȶ����ǽ�������O����N������ˮ���ȶ��Դ��ڰ����ġ�
���ǽ�����Խǿ���⻯��Խ�ȶ����ǽ�������O����N������ˮ���ȶ��Դ��ڰ����ġ�
��ϰ��ϵ�д�
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ
 RCH2OH��NaCl
RCH2OH��NaCl