��Ŀ����
10��ʱ�����ձ��м���0.1mol/L�� NaHCO3��Һ400mL�����ȣ���ø���Һ��pH�������±仯��| �¶ȣ��棩 | 10 | 20 | 30 | 50 | 70 |
| pH | 8.3 | 8.4 | 8.5 | 8.9 | 9.4 |
��2����ͬѧ��Ϊ����ҺpH���ߵ�ԭ����NaHCO3���ȷֽ⣬������Na2CO3�����ƶ�Na2CO3��ˮ��̶�
______������ڡ���С�ڡ���NaHCO3��
��3����ͬѧ��Ϊ��Ҫȷ����������˵��������ֻҪ�Ѽ��Ⱥ����Һ��ȴ��10����ٲⶨ��ҺpH����pH______8.3�����������������=������˵������ȷ����pH______8.3�����������������=������˵������ȷ��
��4����ͬѧ�������ʵ�鷽���Լס���ͬѧ�Ľ��ͽ����жϣ�
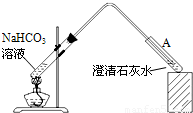
ʵ��װ����ͼ���������NaHCO3��Һ�������Թ�A�в���������˵��______����ס����ҡ����Ʋ���ȷ��
��5����һ�����0.1mol/L�� NaHCO3��Һ�����ձ��м������У���Һ������䣩������pHΪ9.8�����ձ���ȴ�����£���һ��ʱ�䣨��Һ������䣩���pHΪ10.1���ݴ����Ͽ����ж�______����ס����ҡ����Ʋ���ȷ��ԭ����______��
��2��̼������ǿ����������ˮ�⣬��̼���Ƶ�ˮ��̶ȴ���̼�����Ƶ�ˮ��̶ȣ�
��3�������Ⱥ�̼�����Ʋ��ֽ⣬��Һ��Ȼ��̼��������Һ���¶Ȳ�����Һ��pHֵҲ���䣻
��4������̼������A�Թ��������Ƴ��ֳ�����֤����Ӧ�����˶�����̼���壻
��5��������ȴ��������ҺPH�еķ�����Ӧ�������������µ����ʣ�̼����ˮ��̶ȴ���̼�����ƣ���Һ��С��ǿ��
�����1��̼��������ǿ��������ʽ�Σ���ˮ�����Һ�ʼ��ԣ�ˮ�ⷽ��ʽΪ��HCO3-+H2O?H2CO3+OH-��
�ʴ�Ϊ��HCO3-+H2O?H2CO3+OH-��
��2��̼������ǿ����������ˮ�⣬̼���Ƕ�Ԫ���ᣬ��һ������̶�ԶԶ���ڵڶ������룬����̼������ӵĵ�һ��ˮ��̶�ԶԶ���ڵڶ���ˮ��̶ȣ�����̼���Ƶ�ˮ��̶ȴ���̼�����Ƶ�ˮ��̶ȣ�
�ʴ�Ϊ�����ڣ�
��3�������Ⱥ�̼�����Ʋ��ֽ⣬��Һ��Ȼ��̼��������Һ���¶Ȳ�����Һ��PHֵҲ���䣬�ɴ�֤��������ȷ�ģ�PH��8.3����ҺpH���ߵ�ԭ����NaHCO3���ȷֽ⣬������Na2CO3��˵������ȷ��
�ʴ�Ϊ���T������
��4���������NaHCO3��Һ�������Թ�A�в���������˵�����ȷֽ�����˶�����̼���壬֤������ȷ��
�ʴ�Ϊ���ң�
��5����һ�����0.1mol/L�� NaHCO3��Һ�����ձ��м������У���Һ������䣩������pHΪ9.8�����ձ���ȴ�����£���һ��ʱ�䣨��Һ������䣩���pHΪ10.1����Һ��ȴ�����º�pH����8.4��˵����ʵ���������Һ�����������ɣ���������ȷ��
�ʴ�Ϊ���ң���Һ��ȴ�����º�pH����8.4��˵����ʵ���������Һ�����������ɣ�
����������̼�����ƿ�����̼�����Ƶ����ʣ�ʵ��̽���������ʵ���Ʒ�������Ӧ������жϣ�����ˮ��ķ���Ӧ�ã���Ŀ�Ѷ��еȣ�

 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д���08�Ϻ�12У�ڶ�����������A������������ȩ�������������������ʵ�飬
��ͬѧ�������ͼʵ��װ�ý��У���B�Թ��м���
10ml 40%����ȩˮ��Һ��5g�����̾���ͷ�ʯ�Ļ�
������ձ��м�50ml�Ĺ��ͣ���C�Թ��м���
10ml����ˮ��
��֪������ʵķе����±���
���� | ��ȩ | ���� | ���� | ˮ | ֲ���� |
�е� | 20.8�� | 117.9�� | 180������ | 100�� | 175�� |
���¶ȼƵĶ���Ϊ60��D80��ʱ��A���������10�D15�Σ�
�������ȣ��������Թ�C�еõ���ˮ��ҺΪ������Һ����ش��������⣺
��1��д������������ȡ����Ļ�ѧ����ʽ ��
��2�����ձ��зŹ��͵�ԭ���� ��
Ϊ�˱�֤ʵ��ijɹ������ձ���Ҳ������ ������͡��ڹ������ǰ�� ���¶ȼ�ˮ�����λ���ǣ�����ǰ ����� ������¶�Ӧ������ ��
��3����C�Թ����ռ�����ˮ��Һ����ij��������ǣ�
�� ��
�� ��
�� ��
��08�Ϻ�12У�ڶ�����������A������������ȩ�������������������ʵ�飬
��ͬѧ�������ͼʵ��װ�ý��У���B�Թ��м���
10ml 40%����ȩˮ��Һ��5g�����̾���ͷ�ʯ�Ļ�
������ձ��м�50ml�Ĺ��ͣ���C�Թ��м���
10ml����ˮ��
��֪������ʵķе����±���
���� | ��ȩ | ���� | ���� | ˮ | ֲ���� |
�е� | 20.8�� | 117.9�� | 180������ | 100�� | 175�� |
���¶ȼƵĶ���Ϊ60��D80��ʱ��A���������10�D15�Σ�
�������ȣ��������Թ�C�еõ���ˮ��ҺΪ������Һ����ش��������⣺
��1��д������������ȡ����Ļ�ѧ����ʽ ��
��2�����ձ��зŹ��͵�ԭ���� ��
Ϊ�˱�֤ʵ��ijɹ������ձ���Ҳ������ ������͡��ڹ������ǰ�� ���¶ȼ�ˮ�����λ���ǣ�����ǰ ����� ������¶�Ӧ������ ��
��3����C�Թ����ռ�����ˮ��Һ����ij��������ǣ�
�� ��
�� ��
�� ��
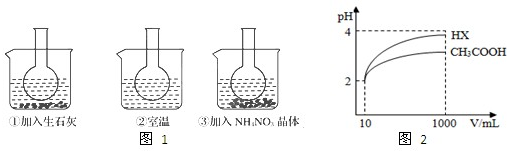
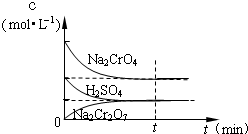 ��25��ʱ����Na2CrO4��Һ�еμ�ϡ���ᣬ��Һ�ɻ�ɫת��Ϊ��ɫ���ڴ�ת�������У�������Ũ�ȱ仯��ͼ��ʾ��д��ת�������з�����Ӧ�����ӷ���ʽ
��25��ʱ����Na2CrO4��Һ�еμ�ϡ���ᣬ��Һ�ɻ�ɫת��Ϊ��ɫ���ڴ�ת�������У�������Ũ�ȱ仯��ͼ��ʾ��д��ת�������з�����Ӧ�����ӷ���ʽ