��Ŀ����
�ۻ�ѧ��ѡ�����ʽṹ�����ʣݣ�15�֣�
��������ѧ����־������2009��ʮ���ѧͻ��֮һ��ʯīϩ���о���Ӧ�÷����ͻ�ơ�ʯīϩ����ԭ�Ӽ��ĺ�ȡ�����ĵ�ѧ���ܡ���ɫ�Ļ�ѧ�ȶ��Ժ�����ѧ�ȶ��ԡ��Ʊ�ʯīϩ������ʯī���뷨����ѧ����������ȡ�ʯīϩ�����ģ�ͼ����ӽṹʾ��ͼ���ң�
��1�������й�ʯīϩ˵����ȷ���ǣߣߣߣߡ�
A��ʯīϩ�Ľṹ����ʯ����
B��ʯīϩ����������ԭ�ӿ��Դ���ͬһƽ��
C��12gʯīϩ���Ҽ���ΪNA
D����ʯī�����ʯīϩ��˷�ʯī�����֮��ķ��Ӽ�������
��2����ѧ����������ǻ�ô���ʯīϩ����Ч����֮һ������Ϊ��ͭ���ܵȽ�����Ͻ𣬺�̼Դ�����Ǽ��顢��Ȳ�������Ҵ���̪ݼ���е�һ�ֻ�������ϡ�
����ԭ���ڻ�̬ʱ����������Ų�ʽΪ���ߣߣߣߣߣߣߣߣߣߣߣߡ�
���Ҵ��е��������ߣ���Ҫԭ���� �ߣߣߣߣߣߣߣߣߣߣߣߡ�
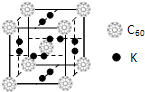
����ͼ�ǽ���ͭ�γɵĽ���������Ͻ����Ļ�ѧʽ�ɱ�ʾΪ���ߣߣߣߣߣߣߣߡ�
�ܺ�̼Դ�����ڷǼ��Է��ӵ��ǣߣߣߣ�a������ b����Ȳ c���� d���Ҵ���
��̪ݼ��̪ݼͭȾ�Ϸ��ӽṹ����ͼ��̪ݼ�����е�ԭ�Ӳ��õ��ӻ���ʽ�У��ߣߣߣߣߣߣߣߡ�
��1��BD��2�֣�ѡ��1����1�֣���ѡ��ѡ���÷֣�
��2����[Ar]3d74s2 ��3�֣� ���Ҵ����Ӽ���γ����������������Ӽ��������2�֣���
��Cu3 Au��Au Cu3 ��2�֣� ��a��b��c��3�֣���sp3��sp2 ��3�֣� ��
����:

 53���ò�ϵ�д�
53���ò�ϵ�д� ��2011?�����ģ����ѧ--ѡ�����ʽṹ������
��2011?�����ģ����ѧ--ѡ�����ʽṹ������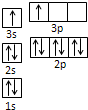
 [��ѧ-ѡ�����ʽṹ������]
[��ѧ-ѡ�����ʽṹ������]