��Ŀ����
����Ŀ��1����������ķ�Ӧ·��������Ϣ��ա�

(1)A�Ľṹ��ʽ��_______��B�Ľṹ��ʽ��______________��
(2)��Ӧ�����õ��Լ���������__________���÷�Ӧ������Ϊ_________________��
(3)��Ӧ�Ļ�ѧ����ʽ��______________��
II.��֪ϩ���뱽��һ�������·�Ӧ���ɷ��������磺

��ϩ������ϳɲ��ϵĻ���ԭ�ϣ������ںϳ�Ӧ�ù㷺��DAP��֬�����˫�ӵ��л��������Ҫ�Ĺ�ҵ��;��
(1)д����ϩ��һ�������·����ۺϷ�Ӧ�Ļ�ѧ����ʽ________________��
(2)��ϩ�뱽��һ�������·�Ӧ������M��N�ȶ��ַ�������������ס��˴Ź������о�����ṹ����Ҫ�����������������ݣ�д��M��N�Ľṹ��ʽ��
M��Ԫ����ɣ�C89.94%��H 10.06%��1H�˴Ź�����5���źš�
N:Ԫ����ɣ�C88.82%��H11.18%��1H�˴Ź�����3���źš�
M��_____��N��_______��
���𰸡� ![]()
 NaOH �Ҵ� ���� ��ȥ��Ӧ
NaOH �Ҵ� ���� ��ȥ��Ӧ ![]() CH2=CHCH3
CH2=CHCH3![]()


�������������л�����ƶϣ�I.��1����Ӧ�ڷ�����ȥ��Ӧ����A�Ľṹ��ʽΪ![]() ��A����ˮ�����ӳɷ�Ӧ����B�Ľṹ��ʽΪ
��A����ˮ�����ӳɷ�Ӧ����B�Ľṹ��ʽΪ ����2����Ӧ�ܷ�����ȥ��Ӧ�����������ƵĴ���Һ�������ȣ���3����Ӧ����±������ˮ�ⷴӦ������Ӧ����ʽΪ��
����2����Ӧ�ܷ�����ȥ��Ӧ�����������ƵĴ���Һ�������ȣ���3����Ӧ����±������ˮ�ⷴӦ������Ӧ����ʽΪ��![]() ��II.��1����ϩ�Ľṹ��ʽΪCH2=CHCH3������̼̼˫���������Ӿ۷�Ӧ���䷴Ӧ����ʽΪCH2=CHCH3
��II.��1����ϩ�Ľṹ��ʽΪCH2=CHCH3������̼̼˫���������Ӿ۷�Ӧ���䷴Ӧ����ʽΪCH2=CHCH3![]() ����2��M��C��H=89.94%/12��10.06%/1=3��4����5���źŷ壬����ʽΪC9H12���ṹ��ʽΪ
����2��M��C��H=89.94%/12��10.06%/1=3��4����5���źŷ壬����ʽΪC9H12���ṹ��ʽΪ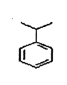 ��N��C��H=88.82%/12��11.18%/1=2��3����3���źŷ壬����ʽΪC12H18���ṹ��ʽΪ
��N��C��H=88.82%/12��11.18%/1=2��3����3���źŷ壬����ʽΪC12H18���ṹ��ʽΪ ��
��

����Ŀ���ɵ�һ�����������ƶϣ����п��������ȶ��ĵ��������ӵ�Ԫ���ǣ� ��
�� | �� | �� | �� | |
��һ�����ܣ�kJ/mol�� | 1681 | 1251 | 1140 | 1008 |
A. �� B. �� C. �� D. ��

