��Ŀ����
6����A��B��C��D����Ԫ�أ����ǵ�ԭ����������������С��18��A��B��ͬһ���ڣ�A�ĵ���ʽΪ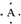 ��Bԭ��L��ĵ���������K���3����0.1mol C�����ܴ������û���2.24L��������״������ͬʱ���ĵ��Ӳ�ṹ�������ԭ�ӵĵ��Ӳ�ṹ��ͬ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��
��Bԭ��L��ĵ���������K���3����0.1mol C�����ܴ������û���2.24L��������״������ͬʱ���ĵ��Ӳ�ṹ�������ԭ�ӵĵ��Ӳ�ṹ��ͬ��D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ����1��д��A��B��C��DԪ�ص����ƣ�A̼��B����Cþ��D����
��2��DԪ��λ�����ڱ��е������ڡ��ڢ�A�壮
��3��A��B�ĵ��ʳ�ַ�Ӧ���ɻ�����ĵ���ʽ��
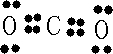 ��
����4��C�����루3�������ɵĻ����ﷴӦ�Ļ�ѧ����ʽ��2Mg+CO2$\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
���� Bԭ��L��ĵ���������K���3������B��ԭ��ԭ�Ӻ������������ֱ�Ϊ2��6��ӦΪOԪ�أ�����A�ĵ���ʽ��֪A���������4�����ӣ�ӦΪ���ڱ��ڢ�A��Ԫ�أ���ԭ������С��O��ӦΪCԪ�أ�0.1mol C�����ܴ������û���2.24L��������״��������C���������2�����ӣ����ӵ�����ԭ�ӵĵ��Ӳ�ṹ��ͬ����CΪMgԪ�أ�D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��DӦΪAlԪ�أ�����Ԫ�ض�Ӧ��ԭ�ӽṹ�Լ��γɻ���������ʽ����⣮
��� �⣺Bԭ��L��ĵ���������K���3������B��ԭ��ԭ�Ӻ������������ֱ�Ϊ2��6��ӦΪOԪ�أ�����A�ĵ���ʽ��֪A���������4�����ӣ�ӦΪ���ڱ��ڢ�A��Ԫ�أ���ԭ������С��O��ӦΪCԪ�أ�0.1mol C�����ܴ������û���2.24L��������״��������C���������2�����ӣ����ӵ�����ԭ�ӵĵ��Ӳ�ṹ��ͬ����CΪMgԪ�أ�D���ӵİ뾶��C���ӵ�С��D������B���ӵĵ��Ӳ�ṹ��ͬ��DӦΪAlԪ�أ�
��1����������ķ�����֪��AΪC��BΪO��CΪMg��DΪAl���ʴ�Ϊ��̼������þ������
��2��DΪAlԪ�أ�ԭ������Ϊ13��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ3��λ�����ڱ��������ڢ�A�壬�ʴ�Ϊ��������A��
��3��A��B�ĵ��ʳ�ַ�Ӧ���ɻ�����ΪCO2������ʽΪ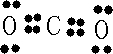 ���ʴ�Ϊ��
���ʴ�Ϊ��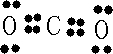 ��
��
��4��CΪMg��þ�������̼��Ӧ�ķ���ʽΪ2Mg+CO2 $\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C���ʴ�Ϊ��2Mg+CO2 $\frac{\underline{\;��ȼ\;}}{\;}$2MgO+C��
���� ���⿼��Ԫ��λ�ýṹ���ʹ�ϵ��Ӧ�ã���Ŀ�Ѷ��еȣ���������ڵ���ʽ�ͻ�ѧ����ʽ����д��ѧϰ��ע�������дҪ�㣮

 ���ɶ��ȫ���ƿؾ�ϵ�д�
���ɶ��ȫ���ƿؾ�ϵ�д�| A�� | ��ţ�䷽�����������ʣ�һ��B��ά���أ������������İ����ᣬ���ǿ�������Щ�����嶼���������� | |
| B�� | ���ۺ���ά�صĻ�ѧʽ��Ϊ��C6H10O5��n������Ϊͬ���칹�� | |
| C�� | ���������ڼ���������ˮ���������Ӧ | |
| D�� | ��������Һ���ж����ЧӦ |
| A�� | �Ͼɵ��ֱ������ | B�� | �����Ľո����ڶѷ� | ||
| C�� | ����ϩ����¶����� | D�� | ������Ʒ�������� |
| A�� | ��Ϊ������Ӧ | B�� | ��Ϊ��ѧ�仯 | ||
| C�� | ��Ϊ�����仯 | D�� | ǰ��Ϊ��ѧ�仯������Ϊ�����仯 |
| A�� | �ೱ��ˮ�帻Ӫ�����Ľ�� | |
| B�� | ����ϴ�Ӽ��㷺ʹ�����ŷ����Ƿ����ೱ����Ҫԭ�� | |
| C�� | �ڷ�յĺ�����������ೱ | |
| D�� | �ೱ�ķ������������ص���Ȼ���� |
| A�� | ����һԪ����HX��HY��HZ�������ƽ�ⳣ�����μ�С����ͬ�����ͬpH�Ķ�Ӧ������Һ�У�������ˮ��������С��NaX��NaY��NaZ | |
| B�� | 25��ʱ��pH=12��NaOH��Һ��pH=12�İ�ˮ�У�c��Na+��=c��NH4+�� | |
| C�� | Ũ�ȷֱ�Ϊ0.1mol•L-1��0.01mol•L-1��CH3COOH��Һ��c��CH3COO-��ǰ���Ǻ��ߵ�10�� | |
| D�� | ��0.01mol•L-1��NH4HSO4��Һ�еμ�NaOH��Һ�����ԣ������������Һ��c��Na+��=c��SO42-����C��H+��=c��OH-�� |
| A�� | 2-��-1-��ϩ | B�� | 2��2-�������� | ||
| C�� | 4-��-2-��Ȳ | D�� | 5��5-����-3-��ϩ |