��Ŀ����
10���Ա�ϩΪԭ�ϣ����Ժϳ�һ������ճ�ϼ���ճ�ϼ��Ľṹ��ʽ�ǣ� ����ϳ�·����ͼ��ʾ��
����ϳ�·����ͼ��ʾ��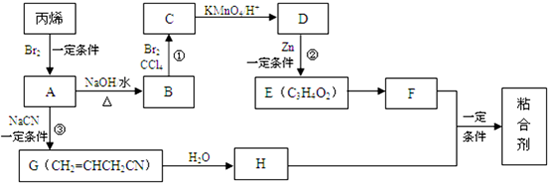
��1��A�ķ���ʽ��C3H5Br��A�����еĹ���������ԭ�Ӻ�̼̼˫��������������ƣ���
��2��H�ܷ����ķ�Ӧ������abcd��
a��ȡ����Ӧ b���ӳɷ�Ӧ c��������Ӧ d����ԭ��Ӧ e�����۷�Ӧ
��3���ϳ�·������Ʒ�Ӧ�ٺ͢ڵ�Ŀ���DZ���̼̼˫����
��4��D������ȥ��Ӧ�������ɺ�̼̼���������ʣ���Ӧ�Ļ�ѧ����ʽ��
 ��
����5��E��F�Ļ�ѧ����ʽ��
 ��
����6���ϳ�ճ�ϼ��Ļ�ѧ����ʽ��
 ��
��
���� A�ķ���ʽ��C3H5Br��˵����ϩ���巢����H��ȡ����Ӧ�����ɵ�AΪCH2=CHCH2Br��A��NaOH��ˮ��Һ�з���ˮ�����ɵ�BΪCH2=CHCH2OH��B��������Ȼ�̼��Һ�����ӳɷ�Ӧ���ɵ�CΪCH2BrCHBrCH2OH��C�е��ǻ��ܹ������Ը�������������Ȼ�������DΪCH2BrCHBrCOOH��D��Zn��һ�������·�����ȥ��Ӧ���ɵ�EΪCH2=CHCOOH����Ʒ�Ӧ�٢ڵ�Ŀ���DZ���̼̼˫���������Ը������������Gˮ�����ɵ�HΪCH2=CHCH2CONH2����ճ�ϼ��Ľṹ��ʽ�ǣ� ��֪��FΪCH2=CHCOOCH3��E��״�����������Ӧ����F���ݴ˷�����
��֪��FΪCH2=CHCOOCH3��E��״�����������Ӧ����F���ݴ˷�����
��� �⣺A�ķ���ʽ��C3H5Br��˵����ϩ���巢����H��ȡ����Ӧ�����ɵ�AΪCH2=CHCH2Br��A��NaOH��ˮ��Һ�з���ˮ�����ɵ�BΪCH2=CHCH2OH��B��������Ȼ�̼��Һ�����ӳɷ�Ӧ���ɵ�CΪCH2BrCHBrCH2OH��C�е��ǻ��ܹ������Ը�������������Ȼ�������DΪCH2BrCHBrCOOH��D��Zn��һ�������·�����ȥ��Ӧ���ɵ�EΪCH2=CHCOOH��Gˮ�����ɵ�HΪCH2=CHCH2CONH2����ճ�ϼ��Ľṹ��ʽ�ǣ� ��֪��FΪCH2=CHCOOCH3��E��״�����������Ӧ����F��
��֪��FΪCH2=CHCOOCH3��E��״�����������Ӧ����F��
��1��A�ķ���ʽ��C3H5Br��˵����ϩ���巢����H��ȡ����Ӧ�����ɵ�AΪCH2=CHCH2Br��A�л�����̼̼˫�����ʴ�Ϊ��̼̼˫����
��2��HΪCH2=CHCH2CONH2���ܹ�������Hԭ�ӵ�ȡ����Ӧ��̼̼˫���ļӳɷ�Ӧ��̼̼˫�������Ը������������̼̼˫���������ļӳɷ�ӦҲ�ǻ�ԭ��Ӧ���ܹ������Ӿ۷�Ӧ�����ܷ������۷�Ӧ���ʴ�Ϊ��abcd��
��3��A��NaOH��ˮ��Һ�з���ˮ�����ɵ�BΪCH2=CHCH2OH��B��������Ȼ�̼��Һ�����ӳɷ�Ӧ���ɵ�CΪCH2BrCHBrCH2OH��C�е��ǻ��ܹ������Ը�������������Ȼ�������DΪCH2BrCHBrCOOH��D��Zn��һ�������·�����ȥ��Ӧ���ɵ�EΪCH2=CHCOOH����Ʒ�Ӧ�٢ڵ�Ŀ���DZ���̼̼˫���������Ը������������
�ʴ�Ϊ������̼̼˫����
��4��DΪCH2BrCHBrCOOH���ܹ���NaOH�Ĵ���Һ�з�����ȥ��Ӧ����CH��CCOONa����ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��5��Gˮ�����ɵ�HΪCH2=CHCH2CONH2����ճ�ϼ��Ľṹ��ʽ�ǣ� ��֪��FΪCH2=CHCOOCH3��E��״�����������Ӧ����F����ѧ����ʽΪ
��֪��FΪCH2=CHCOOCH3��E��״�����������Ӧ����F����ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��6��FΪCH2=CHCOOCH3��HΪCH2=CHCH2CONH2����ͨ���Ӿ۷�Ӧ�ϳ�ճ�ϼ��Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼�����л�����ƶϣ��Ѷ��еȣ����л���ṹ�ı仯��Ϊͻ�ƿ������������ȷ�����ŵ������ǽⱾ��Ĺؼ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| �� | HF | Ka=6.8��10-4 mol•L-1 |
| �� | CH3COOH | Ka=1.7��10-5 mol•L-1 |
| �� | HCN | Ka=6.2��10-10 mol•L-1 |
| �� | H2CO3 | Ka1=4.4��10-7 mol•L-1 Ka2=4.7��10-11 mol•L-1 |
��2��д��H2CO3�ĵ��뷽��ʽH2CO3?HCO3-+H+��HCO3-?CO32-+H+��
��3��д����ѧ����ʽ���������������̼������Һ���2HF+Na2CO3�T2NaF+H2O+CO2��������CO2ͨ��NaCN��Һ��NaCN+H2O+CO2�THCN+NaHCO3��
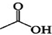 +
+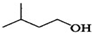 $?_{��}^{ŨH_{2}SO_{4}}$
$?_{��}^{ŨH_{2}SO_{4}}$ +H2O
+H2O| ��Է������� | �ܶ�/��g•cm-3�� | �е�/�� | ˮ���ܽ��� | |
| ���촼 | 88 | 0.8123 | 131 | �� |
| ���� | 60 | 1.0492 | 118 | �� |
| ���������� | 130 | 0.8670 | 142 | ���� |
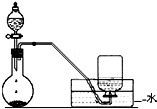
��A�м���4.4g�����촼��6.0g�����ᡢ����Ũ�����2��3Ƭ���Ƭ����ʼ��������A������50min����ӦҺ�������º����Һ©���У��ֱ�������ˮ������̼��������Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬����Ƭ�̣����˳�ȥMgSO4���壬�����������ռ�140��143����֣�������������3.9g��
�ش��������⣺
��1������B�����������������ܣ�
��2����ϴ�ӡ���Һ�����У�Ӧ�����Ȼ���ã����ֲ��d�����ţ���
a��ֱ�ӽ������������ӷ�Һ©�����Ͽڵ���
b��ֱ�ӽ������������ӷ�Һ©�����¿ڷų�
c���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������¿ڷų�
d���Ƚ�ˮ��ӷ�Һ©�����¿ڷų����ٽ��������������Ͽڵ���
��3����ʵ���м�����������Ŀ������ߴ���ת���ʣ�
��4��ʵ���м���������ˮMgSO4��Ŀ���Ǹ���������������
��5������������У�ͼ2������ѡ��װ����ȷ����b�����ţ���
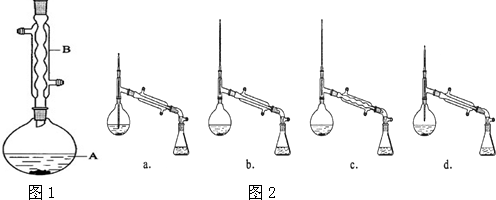
��6���ڽ����������ʱ������130��㿪ʼ�ռ���֣���ʹʵ��IJ���ƫ�ߣ���ߡ��͡�����ԭ���ǻ��ռ�����δ��Ӧ�����촼��
��7����ʵ��IJ�����c�����ţ���a��30% b��40% c��60% d��90%
| A�� | Ũ������ͭ��Ӧ��ȡ�������� | B�� | ̼������ʳ��ˮ��Ӧ��ȡ��Ȳ | ||
| C�� | Ũ��ˮ����ʯ�ҷ�Ӧ��ȡ���� | D�� | Ũ����Ͷ������̷�Ӧ��ȡ���� |
| A�� | 1.5 mol•L-1 | B�� | 2.25 mol•L-1 | C�� | 3 mol•L-1 | D�� | ������ |
| A�� | ��Fe��NO3��2��Ʒ����ϡH2SO4�μ�KSCN��Һ���۲���Һ�Ƿ��죬���Լ���Fe��NO3��2�����Ƿ����������� | |
| B�� | Ũ��ˮ�еμ�FeCl3������Һ���Ƶ�Fe��OH��3���� | |
| C�� | 1 mol FeI2������������Ӧʱת�Ƶĵ�����Ϊ2NA | |
| D�� | ˮ��Һ��Fe2+��H+��SO${\;}_{3}^{2-}$��ClO-���ܴ������� |
| A�� | ���Ƶ���ˮ��������ɫ���ƿ�У������������� | |
| B�� | �����й�Ƭ���廯����������ɫ�Լ�ƿ�� | |
| C�� | �ռ���Һ���ڴ�ĥ�ڲ��������Լ�ƿ�� | |
| D�� | 1mol/L NaCl��Һ���ܳ��ڱ���������ƿ�� |
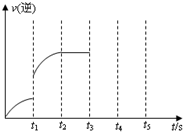 һ���¶��������Ϊ5L���ܱ������з������淴Ӧ��
һ���¶��������Ϊ5L���ܱ������з������淴Ӧ��