��Ŀ����
��14�֣���ѧ��ѧ����������A��B��C��D֮���������ת����ϵ��
A + B �� C + D + H2O���밴Ҫ����գ�
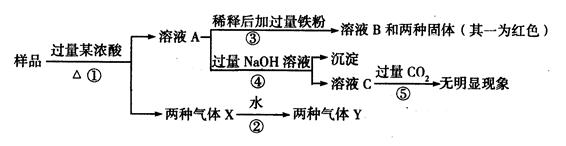
��1����AΪ������Ԫ����ɵĺ�ɫ���嵥�ʣ���B��Ũ��Һ����ʱ������C��D��������,
C��D�����������ʹ����ʯ��ˮ����ǣ���A��B��Ӧ�ķ���ʽ�� ����֪���д̼�����ζ��C�����Cl2����Ư��ijЩ��ɫ���ʣ������������������ʵ���ͨ�뵽Ʒ����Һ�е�ʵ������Ϊ ԭ���� ��
�������������ǵ���ͨ�뵽ˮ�У�Ϊ����֤�������������ij��ȤС���ͬѧ���������Լ��� �� �Ȼ�����Һ �� �Ȼ�������Һ �� ���軯����Һ �� ������Һ
�� Ʒ����Һ �� ���Ը��������Һ
��Cl2������ȡ������Һ�μ���ʢ�� ��ѡ��һ����ţ��Լ����Թ��ڣ��ټ���
��ѡ��һ����ţ��Լ��������������ǣ� ��
��C������ȡ������Һ�μ���ʢ�� ��ѡ��һ����ţ��Լ����Թ��ڣ�����������
�ǣ� ��
��2����A��ˮ�е��ܽ�����¶ȵ����߶����ͣ�BΪ�����ڷǽ������ʣ�C��Ư�۵���Ч�ɷ֣���C����ˮ�ⷴӦ�����ӷ���ʽ�� ��
��3����AΪ���10���ӵ��������뵥��18���ӵ������ӹ��ɵ���ɫ���壬�����ֽ⣬�ֽ���������ּ�������ˮ�����塣����A�������ӵķ�����
�������������̼����ۣ���
A + B �� C + D + H2O���밴Ҫ����գ�
��1����AΪ������Ԫ����ɵĺ�ɫ���嵥�ʣ���B��Ũ��Һ����ʱ������C��D��������,
C��D�����������ʹ����ʯ��ˮ����ǣ���A��B��Ӧ�ķ���ʽ�� ����֪���д̼�����ζ��C�����Cl2����Ư��ijЩ��ɫ���ʣ������������������ʵ���ͨ�뵽Ʒ����Һ�е�ʵ������Ϊ ԭ���� ��
�������������ǵ���ͨ�뵽ˮ�У�Ϊ����֤�������������ij��ȤС���ͬѧ���������Լ��� �� �Ȼ�����Һ �� �Ȼ�������Һ �� ���軯����Һ �� ������Һ
�� Ʒ����Һ �� ���Ը��������Һ
��Cl2������ȡ������Һ�μ���ʢ�� ��ѡ��һ����ţ��Լ����Թ��ڣ��ټ���
��ѡ��һ����ţ��Լ��������������ǣ� ��
��C������ȡ������Һ�μ���ʢ�� ��ѡ��һ����ţ��Լ����Թ��ڣ�����������
�ǣ� ��
��2����A��ˮ�е��ܽ�����¶ȵ����߶����ͣ�BΪ�����ڷǽ������ʣ�C��Ư�۵���Ч�ɷ֣���C����ˮ�ⷴӦ�����ӷ���ʽ�� ��
��3����AΪ���10���ӵ��������뵥��18���ӵ������ӹ��ɵ���ɫ���壬�����ֽ⣬�ֽ���������ּ�������ˮ�����塣����A�������ӵķ�����
�������������̼����ۣ���
��1��C + 2H2SO4(Ũ)  CO2��+2SO2��+2H2O ��2�֣�
CO2��+2SO2��+2H2O ��2�֣�
������1�֣���Cl2+SO2+2H2O��4H++2Cl�D+SO42�D��ʧȥƯ���ԣ�2�֣�
�ڣ�1�֣� �ۣ���ܣ���1�֣� ��Һ���ֺ�ɫ������ɫ����1�֣�
�ݣ���ޣ���1�֣� ��ɫ��ȥ���Ϻ�ɫ��ȥ����1�֣�
��2��ClO��+ H2O HClO + OH�� ��2�֣�
HClO + OH�� ��2�֣�
��3��ȡ����A����ˮ�����Һ���Թ��У������Һ�м���NaOH�����ȣ���ʪ��ĺ�ɫʯ����ֽ���ڹܿڼ����������壬ʯ����ֽ����֤����Һ�к���NH4+�� ������Ũ������飬�а������ɣ� ��2�֣�
 CO2��+2SO2��+2H2O ��2�֣�
CO2��+2SO2��+2H2O ��2�֣�������1�֣���Cl2+SO2+2H2O��4H++2Cl�D+SO42�D��ʧȥƯ���ԣ�2�֣�
�ڣ�1�֣� �ۣ���ܣ���1�֣� ��Һ���ֺ�ɫ������ɫ����1�֣�
�ݣ���ޣ���1�֣� ��ɫ��ȥ���Ϻ�ɫ��ȥ����1�֣�
��2��ClO��+ H2O
 HClO + OH�� ��2�֣�
HClO + OH�� ��2�֣���3��ȡ����A����ˮ�����Һ���Թ��У������Һ�м���NaOH�����ȣ���ʪ��ĺ�ɫʯ����ֽ���ڹܿڼ����������壬ʯ����ֽ����֤����Һ�к���NH4+�� ������Ũ������飬�а������ɣ� ��2�֣�
��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ

 ��Ϊ����������ˣ�Marie Curie�����RaԪ�ص��о����Ȼ��ŵ��������������������ȷ����
��Ϊ����������ˣ�Marie Curie�����RaԪ�ص��о����Ȼ��ŵ��������������������ȷ����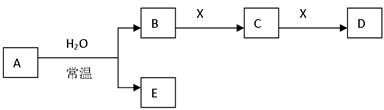
 ij�ֺϽ����ԭ�ӷ�Ӧ�ѵĵ��ȼ���X�ǷǼ��Է��ӵĻ������A��H2O��Ӧ�����ӷ���ʽΪ ��Cת����D�Ļ�ѧ����ʽΪ
ij�ֺϽ����ԭ�ӷ�Ӧ�ѵĵ��ȼ���X�ǷǼ��Է��ӵĻ������A��H2O��Ӧ�����ӷ���ʽΪ ��Cת����D�Ļ�ѧ����ʽΪ 