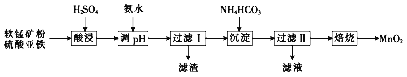
��Ŀ����
����Ŀ����ʽ̼��Ǧ[��ѧʽΪ2PbCO3��Pb(OH)2]������Ǧ������������ԭ�ϡ�
(1) ij�о�С���Ʊ���ʽ̼��Ǧ����Ҫʵ���������£�

�١���Ӧ��������������(CH3COO)2Pb��Pb(OH)2���÷�Ӧ����90�������貢����3 h����������ɵ�����÷�Ӧ�Ļ�ѧ����ʽΪ_______________________________________��
������ʱ����Ҫ�IJ���������©����________��
��������ˮϴ�Ӻ����þƾ�ϴ�ӵ�Ŀ����____________________________________��
(2) Ϊȷ��2PbCO3��Pb(OH)2���ȷֽ����������������ʵ�飺
��ȡһ����(1)���ƵõIJ�Ʒ�������ط�����������������м��ȷֽ�����ò���������������¶ȵı仯����ͼ��ʾ��

��A��B���������ݳ��ķֽ����Ļ�ѧʽΪ______________��E���������Ļ�ѧʽΪ______________����
������ͼ�����ݣ����㲢ȷ��D���������Ļ�ѧʽ(д���������)______________��
���𰸡� 2PbO��2CH3COOH![]() (CH3COO)2Pb��Pb(OH)2 �ձ��������� ��ȥ�������渽�ŵ�ˮ���ٽ�����ٸ��� H2O PbO 1 mol 2PbCO3��Pb(OH)2(����������ʽ��ʾΪ��3PbO��2CO2��H2O)������Ϊ775 g��155 g 2PbCO3��Pb(OH)2�����ʵ���Ϊ0.2 mol�����Ա�ʾΪ����PbO 0.6 mol��CO2 0.4 mol��H2O 0.2 mol�������ȹ�����PbԪ�ص����������������A��B�Ĺ���������m1��3.6 g�����ٵ���0.2 mol H2O��B��D�Ĺ���������m2��8.8 g�����ٵ���0.2 mol CO2��D��E�Ĺ���������m3��8.8 g�����ٵ���0.2 mol CO2��������B��Ļ�ѧʽΪ2PbCO3��PbO����3PbO��2CO2��D��Ļ�ѧʽΪPbCO3��2PbO����3PbO��CO2
(CH3COO)2Pb��Pb(OH)2 �ձ��������� ��ȥ�������渽�ŵ�ˮ���ٽ�����ٸ��� H2O PbO 1 mol 2PbCO3��Pb(OH)2(����������ʽ��ʾΪ��3PbO��2CO2��H2O)������Ϊ775 g��155 g 2PbCO3��Pb(OH)2�����ʵ���Ϊ0.2 mol�����Ա�ʾΪ����PbO 0.6 mol��CO2 0.4 mol��H2O 0.2 mol�������ȹ�����PbԪ�ص����������������A��B�Ĺ���������m1��3.6 g�����ٵ���0.2 mol H2O��B��D�Ĺ���������m2��8.8 g�����ٵ���0.2 mol CO2��D��E�Ĺ���������m3��8.8 g�����ٵ���0.2 mol CO2��������B��Ļ�ѧʽΪ2PbCO3��PbO����3PbO��2CO2��D��Ļ�ѧʽΪPbCO3��2PbO����3PbO��CO2
��������(1)������Ӧ��������������(CH3COO)2PbPb(OH)2���÷�Ӧ����90�桢���貢����3h����������ɵģ���÷�Ӧ�Ļ�ѧ����ʽΪ��2PbO+2CH3COOH![]() (CH3COO)2PbPb(OH)2���ʴ�Ϊ��2PbO+2CH3COOH
(CH3COO)2PbPb(OH)2���ʴ�Ϊ��2PbO+2CH3COOH![]() (CH3COO)2PbPb(OH)2��
(CH3COO)2PbPb(OH)2��
�ڹ���ʱ����Ҫ�IJ���������©�����ձ������������ʴ�Ϊ���ձ�����������
����ϴ����ʱ����ˮϴ�Ӻ����þƾ�ϴ�ӵ�Ŀ���dz�ȥ�����ˮ��ͬʱ���Կ��ٸ���ô���2PbCO3Pb(OH)2�IJ�Ʒ���ʴ�Ϊ����ȥ�������渽�ŵ�ˮ���ٽ�����ٸ��
(2)��1mol2PbCO3Pb(OH)2(����������ʽ��ʾΪ��3PbO2CO2H2O)������Ϊ775g��155g2PbCO3Pb(OH)2�����ʵ���Ϊ0.2mol�����Ա�ʾΪ����PbO0.6mol��CO20.4mol��H2O0.2mol�������ȹ�����PbԪ�ص��������䣬���A��B�Ĺ����У���m1=3.6g�����ٵ���0.2molH2O��B��D�Ĺ����У���m2=8.8g�����ٵ���0.2molCO2��D��E�Ĺ����У���m3=8.8g�����ٵ���0.2molCO2����E������Ĺ���ΪPbO���ʴ�Ϊ��H2O��PbO��
��1mol2PbCO3Pb(OH)2(����������ʽ��ʾΪ��3PbO2CO2H2O)������Ϊ775g��155g2PbCO3Pb(OH)2�����ʵ���Ϊ0.2mol�����Ա�ʾΪ����PbO0.6mol��CO20.4mol��H2O0.2mol�������ȹ�����PbԪ�ص��������䣬���A��B�Ĺ����У���m1=3.6g�����ٵ���0.2molH2O��B��D�Ĺ����У���m2=8.8g�����ٵ���0.2molCO2��D��E�Ĺ����У���m3=8.8g�����ٵ���0.2molCO2�����ԣ�B��Ļ�ѧʽΪ2PbCO3PbO����3PbO2CO2��D��Ļ�ѧʽΪPbCO32PbO����3PbOCO2����D���������Ļ�ѧʽΪPbCO32PbO��3PbOCO2��

 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�