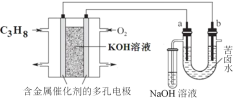
��Ŀ����
����Ŀ����1�������ϻ����CSH���������������ĺ����л�������ʺʹ���������ơ�ijЩ���������д����������ζ��������ʳ���㾫��
�� �ϻ����CSH���ĵ���ʽ��________________��
�� ��д������C2H5SH�����Ʒ�Ӧ�Ļ�ѧ����ʽ________________��
��2��д���������ʵĽṹ��ʽ
��2,3-����-4-�һ�����
_______________________________��
��֧��ֻ��һ���һ���ʽ����С������
______________________________________��
��3��ij������Է�������Ϊ56������̼����������Ϊ85.7%�������������Ϊ14.3%�� ������ķ���ʽ��__________�������ж���ͬ���칹�壬����һ����ʹBr2/CCl4��Һ��ɫ�Ҿ���˳���칹����д���䷴ʽ�ṹ�Ľṹ��ʽ____________________��
��4���߾��� �ĵ���Ϊ__________________��
�ĵ���Ϊ__________________��
A��CH2=CH�CCH2CH3 B��CH2=C(CH3)2
C��CH2=CH2 D��CH2=CHCH3
���𰸡� ![]() 2Na + 2C2H5SH �� 2C2H5SNa + H2��
2Na + 2C2H5SH �� 2C2H5SNa + H2�� 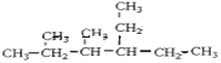 CH��CH2CH3��3 C4H8
CH��CH2CH3��3 C4H8 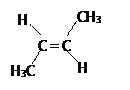 BCD
BCD
��������(1)�����ϻ�(�CSH)�������������ĺ����л�������ʺʹ���������ơ�ijЩ���������д����������ζ��������ʳ���㾫��
�� �ϻ�(�CSH)�ĵ���ʽ���ǻ����ƣ�����ʽΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
������(C2H5SH)���������Ҵ����ƣ����Ʒ�Ӧ�Ļ�ѧ����ʽΪ2Na + 2C2H5SH �� 2C2H5SNa + H2�����ʴ�Ϊ��2Na + 2C2H5SH �� 2C2H5SNa + H2����
(2)![]() �Ľṹ��ʽΪ
�Ľṹ��ʽΪ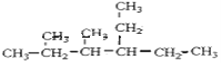 ���ʴ�Ϊ��
���ʴ�Ϊ��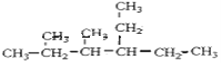 ��
��
![]() ��֧��ֻ��һ���һ���ʽ����С����������������5��̼ԭ�ӣ��ṹ��ʽΪCH(CH2CH3)3���ʴ�Ϊ��CH(CH2CH3)3��
��֧��ֻ��һ���һ���ʽ����С����������������5��̼ԭ�ӣ��ṹ��ʽΪCH(CH2CH3)3���ʴ�Ϊ��CH(CH2CH3)3��
(3)ij������Է�������Ϊ56������̼����������Ϊ85.7%�������������Ϊ14.3%�� ������ķ���ʽ��__________�������ж���ͬ���칹�壬����һ����ʹBr2/CCl4��Һ��ɫ�Ҿ���˳���칹����д���䷴ʽ�ṹ�Ľṹ��ʽ____________________��
̼����������Ϊ85.7%�������������Ϊ14.3%����N(C)��N(H)= ![]() ��
�� ![]() =1��2���ʸ��������ʽΪCH2����ʵ��ʽΪ��CH2����Է�������Ϊ56�������Ϊ(CH2)n����14n=56�����n=4���ʸ÷���ʽΪC6H8������һ����ʹBr2/CCl4��Һ��ɫ�Ҿ���˳���칹���ʸ����ķ�ʽ�ṹ�Ľṹ��ʽΪ
=1��2���ʸ��������ʽΪCH2����ʵ��ʽΪ��CH2����Է�������Ϊ56�������Ϊ(CH2)n����14n=56�����n=4���ʸ÷���ʽΪC6H8������һ����ʹBr2/CCl4��Һ��ɫ�Ҿ���˳���칹���ʸ����ķ�ʽ�ṹ�Ľṹ��ʽΪ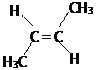 ���ʴ�Ϊ��C4H8��
���ʴ�Ϊ��C4H8�� ��
��
(4)������������ֻ��̼ԭ�Ӳ�����̼̼˫���ṹ�ĸ߾��������ǡ���˫�����ĸ�̼����˫��������̼�����߶Ͽ���Ȼ����պϣ�������˫����������˸߾���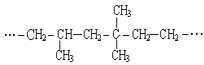 �ĵ���ΪCH2=C(CH3)2��CH2=CH2��CH2=CH2
�ĵ���ΪCH2=C(CH3)2��CH2=CH2��CH2=CH2
CH2=CHCH3���ʴ�Ϊ��BCD��
���磺�Ӿ۲���ĵ����ƶϣ��ٷ����ڵ�������ֻ������̼ԭ��(������ԭ��)�ĸ߾����ϳɵ����Ϊһ�֣����������պϼ��ɣ��ڷ���������̼��Ϊ4��̼ԭ�ӣ���̼̼˫���ṹ���䵥���Ϊ���֣��������м�Ͽ����ٷֱ���������պϼ��õ��壻�۷���������̼��Ϊ6��̼ԭ�ӣ�����̼̼˫���ṹ������Ϊ����(����ϩ���Ͷ�ϩ��)��