��Ŀ����
8����������һ����Ҫ���л���Ԫ�ᣬ��Ҫ����������������ĭ���ϼ��������ܼ��ȣ���;ʮ�ֹ㷺��������Ĵ�ͳ����ɫ�ϳɷ�ԭ���ֱ���ͼ1��
��ɫ�ϳ��������£�
��100mL������ƿ�м���2.00g�����������ƺͲ���ϳɣ���34.0mL 30%��H2O2�ʹ��ӣ������½���15-20min����6.00g����ϩ����û���װ�ã���ͼ2�����������پ��ҽ��貢���Ȼ�������Ӧ�����л����¶Ƚ��������ߣ�����l.5h�õ��ȵĺϳ�Һ��
ע���ټ������ɫ���壬�۵�153.0-153.1�棬�����ھƾ�������ϩ����ɫҺ�壬������ˮ�������Ҵ���
�ڼ���������ˮ�е��ܽ��������ͼ3��
��1���Աȴ�ͳ����ɫ�Ʊ���ԭ������ͳ�Ƹ���������̭����Ҫԭ���ǣ�Ũ�������ǿ��ʴ�ԣ����豸��ʴ���أ���Ӧ�л������ж��ĵ��������Ⱦ������
��2����ɫ�ϳ�ʱ������ͱ�����پ��ҽ����ԭ���ǣ�����ϩ������ˮ�����ٽ��������ڷ�Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
��3������ʱ�����õ��������������á���ȫƿ������ƿ������©������������Ƥ�������ܵȣ�ָ������ƿ����ͨ��ƿ�������������㲻֮ͬ��������ƿ����֧�ܡ�����ƿƿ�ڱ���ƿ��
��4�����Ⱥϳ�Һ�з���õ����������IJ��裺��ȴ�ᾧ�����ˣ�ϴ�ӣ��������������ѡ����ʵ�ϴ�Ӽ���B��
A������ˮ B����ˮ����� C���Ҵ� D����Һ
��5������δ��Ӧ��Ļ���ϩ�ķ����ǣ���Һ��
��6����ëϸ�ֱܷ�ȡ��ͳ������ɫ���Ʊ���Ʒ�ľƾ���Һ���ֱ��������ֽ��ĩ��1cm����������ʵ�飬��ɫ����ͼ4��ʾ����ʵ���֪����ͳ���ƵõIJ�Ʒ���ȱ���ɫ���ߣ�
���� ��1��Ũ�������ǿ��ʴ�ԣ���Ӧ�л������ж��ĵ��������Ⱦ������
��2������ϩ������ˮ�����ٽ��������ڷ�Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
��3�����ݳ���װ��ѡ������������ƿ����ͨ��ƿ�������������㲻ͬΪ������ƿ����֧�ܣ�����ƿƿ�ڱ���ƿ��
��4����Һ�еõ����ʹ���ķ�������ȴ�ᾧ�����ˣ�ϴ�ӣ������������ھƾ����ܽ�����¶ȱ仯�ϴ�Ҫ��С��ϴ�ӵ��µ���ʧ��
��5������ϩ������ˮ����ȡ��Һ�������з�����գ�
��6������ʵ�������ͼ�仯��֪���ô�ͳ�����в���ʵ�飬�ްߵ㣬��ɫ���Ʊ��аߵ㣬˵����ͳ���õ���Ʒ�Ĵ��ȴ�
��� �⣺��1����Ӧ�����Ὣ����������Ϊ�����ᡢ�������⽫����ϩ����Ϊ�����ᣬ�������������ã�����ɫ�Ʊ���ȣ�Ũ�������ǿ��ʴ�ԣ����豸��ʴ���أ���Ӧ�л������ж��ĵ��������Ⱦ������
�ʴ�Ϊ��Ũ�������ǿ��ʴ�ԣ����豸��ʴ���أ���Ӧ�л������ж��ĵ��������Ⱦ������
��2�����ڻ���ϩ������ˮ�����ٽ��������ڷ�Ӧ���ֽӴ����ӿ췴Ӧ���ʣ��ϳ�ʱ������پ��ҽ��裬
�ʴ�Ϊ������ϩ������ˮ�����ٽ��������ڷ�Ӧ���ֽӴ����ӿ췴Ӧ���ʣ�
��3������ʱ�����õ��������������á���ȫƿ������ƿ������©������������Ƥ�������ܵȣ�����ƿ����ͨ��ƿ�������������㲻֮ͬ��Ϊ������ƿ����֧�ܡ�����ƿƿ�ڱ���ƿ��
�ʴ�Ϊ������©��������ƿ����֧�ܡ�����ƿƿ�ڱ���ƿ��
��4�����Ⱥϳ�Һ�з���õ����������IJ���Ϊ��ȴ�ᾧ�������������ھƾ����þƾ�ϴ�ӻ��ܽ����ʧ�ϴ����ܽ�����¶ȱ仯�ϴ��ñ�ˮ�����ϴ�ӣ����Լ�С��ϴ�ӵ��µ���ʧ��
�ʴ�Ϊ����ȴ�ᾧ��B��
��5�����ڻ���ϩ������ˮ�����Բ�ȡ��Һ�������з�����գ����õ���Ҫ����Ϊ��Һ©����
�ʴ�Ϊ����Һ��
��6������ʵ�������ͼ�仯��֪���ô�ͳ�����в���ʵ�飬�ްߵ㣬��ɫ���Ʊ��аߵ㣬˵����ͳ���õ���Ʒ�Ĵ��ȴ�
�ʴ�Ϊ����ͳ���ƵõIJ�Ʒ���ȱ���ɫ���ߣ�
���� ���⿼���л�����Ʊ�ʵ�飬�漰�Է�Ӧԭ���ķ�����������ʶ�����ʷ����ᴿ���Բ�����װ�õķ������ۡ����ʼ���ȣ��Ѷ��еȣ�

 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д� Сѧ��ѧ������ѿڶ���ϵ�д�
Сѧ��ѧ������ѿڶ���ϵ�д� ������Ӧ�������������ϵ�д�
������Ӧ�������������ϵ�д�| A�� | H2O+H2O?H3O++OH- | B�� | NaHSO4�TNa++HSO4- | ||
| C�� | NaHS�TNa++HS- | D�� | HCO3-+H2O?H3O++CO32- |
| A�� | һ����2��1 | B�� | ��������2��1 | ||
| C�� | ֻ��ͬ��ͬѹ��Ϊ2��1 | D�� | ֻ�ڱ�״����Ϊ2��1 |
| A�� | BOH����ˮ������뷽��ʽ��BOH�TB++OH- | |
| B�� | ����һ������������Һ��Ϻ�pH=7����c��A- ��=c��B+�� | |
| C�� | ��0.1mol/L BA��Һ�У�c��B+����c��A- ����c��OH- ����c��H+�� | |
| D�� | ����0.1mol/L BOH��Һϡ����0.001mol/L������Һ��pH=9 |
| A�� | ������̼������Һ��Ӧ��2H++CO${\;}_{3}^{2-}$�TCO2��+H2O | |
| B�� | ������Һ������������ͭ��Ӧ��CH3COOH+OH-��CH3COO-+H2O | |
| C�� | ��ȩ��Һ��������������Һ���ȣ�HCHO+4[Ag��NH3��2]++4OH-$\stackrel{��}{��}$ CO${\;}_{3}^{2-}$+2NH${\;}_{4}^{+}$+4Ag��+6NH3+2H2O | |
| D�� | ��������Һ��ͨ������������̼��C6H5O-+CO2+H2O��C6H5OH+CO32- |
 ����ͼ�ش��������⣺
����ͼ�ش��������⣺

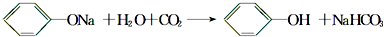 ��
��