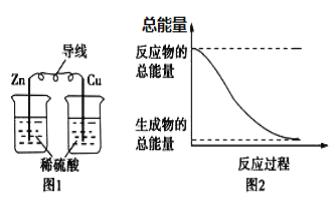
��Ŀ����
����Ŀ��һ���¶��£������������Ϊ1.0L�ĺ����ܱ������з�����Ӧ��2CH3OH��g��CH3OCH3��g��+H2O��g��
������� | �¶ȣ��棩 | ��ʼ���ʵ�����mol�� | ƽ�����ʵ�����mol�� | |
CH3OH��g�� | CH3OCH3��g�� | H2O | ||
�� | 387 | 0.20 | 0.080 | 0.080 |
�� | 387 | 0.40 | ||
�� | 207 | 0.20 | 0.090 | 0.090 |
����˵����ȷ���ǣ�������
A.�÷�Ӧ������ӦΪ���ȷ�Ӧ
B.�ﵽƽ��ʱ���������е�CH3OH����������������е�С
C.�������з�Ӧ����ƽ������ʱ����������еij�
D.����ʼʱ���������г���CH3OH 0.1mol��CH3OCH3 0.15mol��H2O 0.10mol����Ӧ��������Ӧ�������
���𰸡�AD
���������⣺A����������ƽ��ʱc��CH3OCH3��=c��H2O��= ![]() =0.080mol/L��c��CH3OH��=
=0.080mol/L��c��CH3OH��= ![]() =0.04mol/L���������л�ѧƽ�ⳣ��K1=
=0.04mol/L���������л�ѧƽ�ⳣ��K1= ![]() =4����������ƽ��ʱc��CH3OCH3��=c��H2O��=
=4����������ƽ��ʱc��CH3OCH3��=c��H2O��= ![]() =0.090mol/L��c��CH3OH��=
=0.090mol/L��c��CH3OH��= ![]() =0.02mol/L����ѧƽ�ⳣ��K2=
=0.02mol/L����ѧƽ�ⳣ��K2= ![]() =20.25��4�����Խ����¶ȣ���ѧƽ�ⳣ������Ӧ������Ӧ�����ƶ���������Ӧ�Ƿ��ȷ�Ӧ����A��ȷ��
=20.25��4�����Խ����¶ȣ���ѧƽ�ⳣ������Ӧ������Ӧ�����ƶ���������Ӧ�Ƿ��ȷ�Ӧ����A��ȷ��
B�����������£��������൱����������Ļ����ϼ�ѹ�������ڸ÷�Ӧ�Ƿ�Ӧǰ�������������ķ�Ӧ�����ƽ�ⲻ�ƶ��������������е�CH3OH����������������е���ȣ���B����
C���������е��¶ȱ�����III���¶ȸߣ��¶�Խ�߷�Ӧ����Խ�죬�ﵽƽ������ʱ��Խ�̣���C����
D��c��CH3OH��=0.1mol/L��c��CH3OCH3 ��=0.15mol/L��c��H2O��=0.10mol/L��Ũ����= ![]() =1.5��4��ƽ��������Ӧ�����ƶ�����D��ȷ��
=1.5��4��ƽ��������Ӧ�����ƶ�����D��ȷ��
��ѡAD��