��Ŀ����
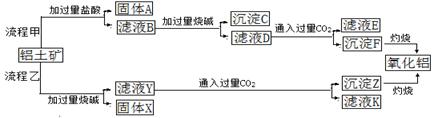
����ʯ����Ҫ�ɷ�ΪK2SO4��Al2(SO4)3��2Al2O3��6H2O�������������Fe2O3���ʡ�ijУ�о�С����������ʯ�Ʊ������������������£�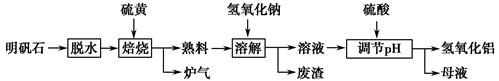
(1)�����ա������з�Ӧ�Ļ�ѧ����ʽΪ Al2(SO4)3��
Al2(SO4)3�� S
S
 Al2O3��
Al2O3�� ________����
________����
(2)������Һ�ͷ����IJ�����________�����ܽ⡱ʱ��Ӧ�����ӷ���ʽΪ_________________________________��
(3)������pH������ˡ�ϴ��Al(OH)3������֤��������ϴ�Ӹɾ���ʵ�������������________��
(4)��ĸҺ���пɻ��յ�������________��
(5)�������ա�������ǡ������48 g��ƣ������������տɵõ�________ g����������
��(1)2��3��2��9��SO2��(2)���ˡ�Al2O3��2OH��===2AlO2����H2O��(3)ȡ���һ��ϴ�ӵ���Һ���Թ��У��μ�BaCl2��Һ�����ް�ɫ�������ɣ���֤����ϴ�Ӹɾ���(4)K2SO4��Na2SO4��(5)468
����

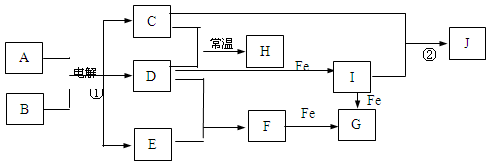
��ҵ����ij����������Cu2O��Al2O3��Fe2O3��SiO2����ȡͭ�IJ����������£�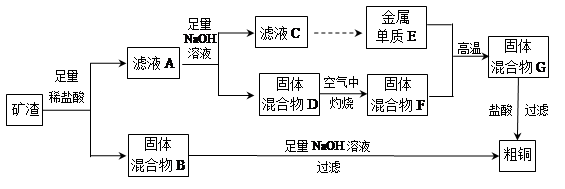
��֪��Cu2O+2H+�TCu+Cu2++H2O
��1����������B������������Һ��Ӧ�Ļ�ѧ����ʽΪ ��
��2����ҺA����Ԫ�صĴ�����ʽֻ��ΪFe2+��������
���漰�����ӷ���ʽΪ ��
��������дCu2O������ķ�Ӧ����������ҺA��Fe2+���Լ�Ϊ �����Լ����ƣ���
��3������ҺC�е���Ԫ���Գ�����ʽ��������ѡ�õ�����Լ�Ϊ __ ������ţ���
| A������������Һ | B��������Һ | C����ˮ | D��������̼ |
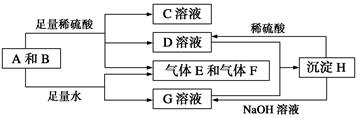

 B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�
B+H�ܷų��������ȣ��÷�Ӧ��Ӧ��������ĺ��ӣ�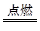 2F+D��F��EԪ�ص���������Ϊ60%��
2F+D��F��EԪ�ص���������Ϊ60%��