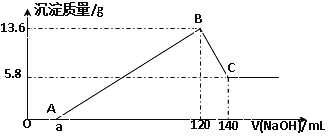
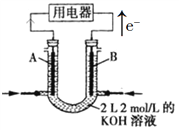
题目内容
【题目】铜及其化合物在生产、生活中有广泛应用,铜在化合物中的常见化合价有+1价、+2 价。已知Cu2O与稀硫酸反应,溶液呈蓝色。
(1)将少量铜丝放入适量的稀硫酸中,温度控制在50℃,加入H2O2,反应一段时间后,升温到60℃,再反应一段时间后可制得硫酸铜,该反应的离子方程式为_______________。温度控制在50℃-60℃的两个原因除了加快反应速率外,还有____________________。在CuSO4溶液中加入一定量的Na2SO3和NaCl 溶液加热,生成CuCl沉淀,写出生成CuCl的离子方程式________________________________。
(2)现向Cu、Cu2O 和CuO组成的混合物中,加入1L 0.6 mol/L HNO3溶液恰好使混合物溶解,同时收集到2240 mL NO 气体(标准状况)。Cu2O跟稀硝酸反应的离子方程式_______________________________。若将上述混合物用足量的H2加热还原,所得到固体的质量为___________g。若混合物中含0.1mol Cu,将该混合物与稀硫酸充分反应,至少消耗H2SO4的物质的量为_____________。
【答案】 Cu+H2O2+2H+=Cu2++2H2O 同时防止H2O2 分解 2Cu2++2Cl-+SO32-+H2O![]() 2CuCl↓+SO42-+2H+ 3Cu2O+14H++2NO3-=6Cu2++2NO↑+7H2O 16 0.1mol
2CuCl↓+SO42-+2H+ 3Cu2O+14H++2NO3-=6Cu2++2NO↑+7H2O 16 0.1mol
【解析】(1).少量铜丝放入适量的稀硫酸中,铜丝与稀硫酸不反应,但加入H2O2后由于双氧水具有强氧化性,在酸性条件下可以把铜氧化成二价铜离子,反应的离子方程式为:Cu+2H++H2O2=Cu2++2H2O;因为H2O2在较高温度时容易分解,所以温度控制在50℃-60℃,可以防止H2O2分解;在CuSO4溶液中加入一定量的Na2SO3和NaCl溶液加热,生成CuCl沉淀,铜元素的化合价降低,则SO32被Cu2+氧化为SO42,反应物除Cu2+、Cl、SO32外,还有H2O,产物有H+,根据得失电子守恒和原子守恒配平得:2Cu2++2Cl-+SO32-+H2O![]() 2CuCl↓+SO42-+2H+,故答案为:Cu+2H++H2O2=Cu2++2H2O;同时防止H2O2分解;2Cu2++2Cl-+SO32-+H2O
2CuCl↓+SO42-+2H+,故答案为:Cu+2H++H2O2=Cu2++2H2O;同时防止H2O2分解;2Cu2++2Cl-+SO32-+H2O![]() 2CuCl↓+SO42-+2H+;
2CuCl↓+SO42-+2H+;
(2).稀硝酸可把+1价的Cu+氧化为+2价的Cu2+,自身被还原为NO,根据得失电子守恒和原子守恒,该反应的离子方程式为:3Cu2O+14H++2NO3-=6Cu2++2NO↑+7H2O;HNO3的物质的量为0.6mol,其中作氧化剂的HNO3被还原为NO,其物质的量为2.24L÷22.4L/mol=0.1mol,则起酸性作用的HNO3为:0.6mol-0.1mol=0.5mol,这部分HNO3转化为了Cu(NO3)2,根据原子守恒可知,原化合物中Cu原子的物质的量与起酸性作用的HNO3的物质的量之比为1:2,所以H2还原最终得到的Cu的质量为:0.5mol÷2×64g/mol=16g;若混合物中含0.1 mol Cu,根据得失电子守恒得:2×0.1mol+2×n(Cu2O)=3×0.1mol,n(Cu2O)=0.05mol,根据题目所给信息,Cu2O与稀硫酸反应,溶液呈蓝色,说明生成了Cu2+,离子方程式为:Cu2O+2H+=Cu2++Cu+H2O,则0.05mol Cu2O完全反应需要H2SO40.05mol, n(CuO)=0.25mol-0.1mol-2×0.05mol=0.05mol,0.05molCuO 完全反应需要H2SO40.05mol,所以共消耗H2SO4的物质的量为0.1mol,故答案为:3Cu2O+14H++2NO3-=6Cu2++2NO↑+7H2O;16;0.1mol。

 天天练口算系列答案
天天练口算系列答案【题目】CO、CO2是火力发电厂释放出的主要尾气,为减少对环境造成的影响,发电厂试图采用以下方法将其资源化利用,重新获得燃料或重要工业产品。
(1)CO 与Cl2在催化剂的作用下合成光气(COCl2)。某温度下,向2L的密闭容器中投入一定量的CO和Cl2,在催化剂的作用下发生反应:CO(g)+Cl2(g)![]() COCl2(g) ;△H=akJ/mol 反应过程中测定的部分数据如下表:
COCl2(g) ;△H=akJ/mol 反应过程中测定的部分数据如下表:
t/min | n (CO) /mol | n(Cl2)/mol |
0 | 1.20 | 0.60 |
1 | 0.90 | |
2 | 0.80 | |
4 | 0.20 |
①反应1~2min末的平均速率v(COCl2)=________mol/(L·min)。
②在2min~4min间,vCl2正_______vCO逆 (填“>”、“=”或“<”),该温度下K=________。
③已知X、L可分别代表温度或压强,下图表示L一定时,CO的转化率随X的变化关系。
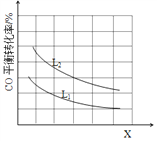
X代表的物理量是___________;a_______0 (填“>”,“=”,“<”)。
(2)在催化剂作用下NO和CO转化为无毒气体:2CO(g) +2NO(g)![]() 2CO2(g) +N2(g);△H=-748kJ·mol-1
2CO2(g) +N2(g);△H=-748kJ·mol-1
①一定条件下,单位时间内不同温度下测定的氮氧化物转化率如图1所示。温度高于710K时,随温度的升高氮氧化物转化率降低的原因可能是________________________________________________。
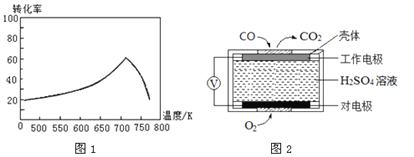
②已知: 测定空气中NO和CO含量常用的方法有两种:
方法1:电化学气敏传感器法。其中CO 传感器的工作原理如图2 所示,则工作电极的反应式为____________。
方法2:氧化还原滴定法。用H2O2溶液吸收尾气、将氮氧化物转化为强酸,用酸碱中和滴定法测定强酸依度。写出NO与H2O2溶液反应的离子方程式:____________。