��Ŀ����
����Ŀ����һ��������Mg-Al�Ͻ�Ͷ��100mLһ�����ʵ���Ũ�ȵ�ijHCl��Һ�У���ַ�Ӧ����Ӧ�����Һ����μ���һ�����ʵ���Ũ�ȵ�NaOH��Һ�����ɳ���������������NaOH��Һ�������ϵ����ͼ���ش��������⣺
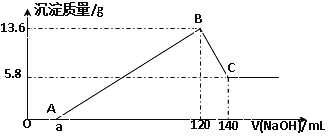
��1��д��OA�κ�BC�η�Ӧ�����ӷ���ʽ��
OA��________________________________ �� BC��_____________________________��
��2��ԭMg-Al�Ͻ��������_____________��
��3��ԭHCl��Һ�����ʵ���Ũ����________________��
��4������NaOH��Һ�����ʵ���Ũ����____________��
��5��a��ֵ��____________��
���𰸡� H++OH-=H2O Al(OH)3+OH-=AlO2-+2H2O 5.1�� 6mol/L 5mol/L 20
����������1����ͼ��֪��OA��û�г������ɣ�˵��ԭ��Һ�������ܽ�Mg��Al��������ʣ�࣬��ʱ�����ķ�ӦΪ��HCl+NaOH=NaCl+H2O����Ӧ�����ӷ���ʽΪ��H++OH-=H2O��BC���������������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ�����ӷ���ʽΪ��Al(OH)3+OH-=AlO2-+2H2O����2��������þ�������������ƣ���ͼ��֪��������þ������Ϊ5.8g�����ʵ�����5.8g��58g/mol��0.1mol������þԭ���غ��֪����þ��������0.1mol��24g/mol��2.4g������������������Ϊ13.6g-5.8g=7.8g�������ʵ���Ϊ7.8g��78g/mol=0.1mol��������ԭ���غ��֪��������������0.1mol��27g/mol��2.7g������ԭMg-Al�Ͻ��������2.4g+2.7g��5.1g����3����Al(OH)3+OH-=AlO2-+2H2O��֪BC������������ȫ�ܽ�������������Ϊ0.1mol������������Һ���Ϊ140mL��120mL��20mL������c��NaOH��=0.1mol��0.02L=5mol/L��B��ʱ���������ʱ��Һ����ΪNaCl��������Ԫ���غ㣬n��NaCl��=n��NaOH��=0.12L��5mol/L=0.6mol��������Ԫ���غ�n��HCl��=0.6mol������������ʵ���Ũ��Ϊ0.6mol��0.1L=6mol/L����4���������Ϸ�����֪����NaOH��Һ�����ʵ���Ũ����5mol/L����5������0.1mol������þ��0.1mol�������������������Ʒֱ���0.2mol��0.3mol��������0.5mol����AB����������������Һ�������0.5mol��5mol/L��0.1L��100mL������a��120��100��20��

����Ŀ�����и������ʲ��ܰ�![]() ��
��![]() �� ��ʾ��Ӧһ�������ϵת������
�� ��ʾ��Ӧһ�������ϵת������ ![]()
ѡ�� | a | b | c |
A | SiO2 | Na2SiO3 | H2SiO3 |
B | AlCl3 | Al(OH)3 | NaAlO2 |
C | Fe | Fe(OH)3 | Fe2O3 |
D | MgCl2 | Mg(OH)2 | MgO |
A. A B. B C. C D. D
