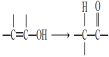ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΚœ≥…ΖΦœψ»≤Μ·ΚœΈοΒΡΖΫΖ®÷°“Μ «‘Ύ¥ΏΜ·ΧθΦΰœ¬Θ§Κ§»≤«βΒΡΖ÷Ή””κδε±ΫΖΔ…ζΖ¥”ΠΘ§»γΘΚ
ΦΉ ““ ±ϊ
ΗυΨί…œ ΫΘ§«κΜΊ¥πΘΚ
Θ®1Θ©ΦΉΒΡΖ÷Ή” Ϋ «___ΘΜ±ϊΡήΖΔ…ζΒΡΖ¥”Π «Θ®―ΓΧνΉ÷ΡΗΘ©___ΓΘ
aΘ°»Γ¥ζΖ¥”Π bΘ°Φ”≥…Ζ¥”Π cΘ°Υ°ΫβΖ¥”Π dΘ°œϊ»ΞΖ¥”Π
Θ®2Θ©“‘±ΫΈΣ‘≠Νœ…ζ≥…““ΒΡΜ·―ßΖΫ≥Χ Ϋ «____ΓΘ
Θ®3Θ©““”κNaOH»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ_____ΓΘ
Θ®4Θ©”…±ϊ÷Τ±Η ![]() ΒΡΖ¥”ΠΧθΦΰ «_______ΓΘ
ΒΡΖ¥”ΠΧθΦΰ «_______ΓΘ
Θ®5Θ©ΖϊΚœœ¬Ν–ΧθΦΰΒΡ±ϊΒΡΆ§Ζ÷“λΙΙΧε”–__÷÷Θ®≤ΜΑϋά®Υ≥Ζ¥“λΙΙΘ©ΓΘΔΌΖ÷Ή”÷–≥ΐ±ΫΜΖΆβ≤ΜΚ§ΤδΥϊΜΖΉ¥ΫαΙΙΘΜΔΎ±ΫΜΖ…œ÷Μ”–2Ηω»Γ¥ζΜυΘ§«“Τδ÷–“ΜΗω «»©ΜυΓΘ
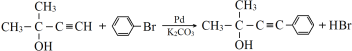
ΓΨ¥πΑΗΓΩC5H8O abd ![]() +Br2
+Br2 ![]() +HBr
+HBr ![]() +2NaOH
+2NaOH ![]() +NaBr+ H2O ≈®ΝρΥαΓΔΦ”»» 24
+NaBr+ H2O ≈®ΝρΥαΓΔΦ”»» 24
ΓΨΫβΈωΓΩ
Θ®1Θ© ΒΡΖ÷Ή” Ϋ «C5H8OΓΘ¥πΑΗΈΣΘΚC5H8OΘΜ
ΒΡΖ÷Ή” Ϋ «C5H8OΓΘ¥πΑΗΈΣΘΚC5H8OΘΜ
 Κ§”–τ«ΜυΓΔΧΦΧΦΥΪΦϋΚΆ±ΫΜυΘ§τ«ΜυΡήΖΔ…ζ»Γ¥ζΖ¥”ΠΓΔœϊ»ΞΖ¥”ΠΘ§ΧΦΧΦΥΪΦϋΡήΖΔ…ζΦ”≥…Ζ¥”ΠΘ§±ΫΜυΡήΖΔ…ζ»Γ¥ζΖ¥”ΠΓΔΦ”≥…Ζ¥”ΠΘ§Υυ“‘ΗΟ”–ΜζΈοΡήΖΔ…ζ»Γ¥ζΖ¥”ΠΓΔΦ”≥…Ζ¥”ΠΓΔœϊ»ΞΖ¥”ΠΓΘ¥πΑΗΈΣΘΚabdΘΜ
Κ§”–τ«ΜυΓΔΧΦΧΦΥΪΦϋΚΆ±ΫΜυΘ§τ«ΜυΡήΖΔ…ζ»Γ¥ζΖ¥”ΠΓΔœϊ»ΞΖ¥”ΠΘ§ΧΦΧΦΥΪΦϋΡήΖΔ…ζΦ”≥…Ζ¥”ΠΘ§±ΫΜυΡήΖΔ…ζ»Γ¥ζΖ¥”ΠΓΔΦ”≥…Ζ¥”ΠΘ§Υυ“‘ΗΟ”–ΜζΈοΡήΖΔ…ζ»Γ¥ζΖ¥”ΠΓΔΦ”≥…Ζ¥”ΠΓΔœϊ»ΞΖ¥”ΠΓΘ¥πΑΗΈΣΘΚabdΘΜ
Θ®2Θ©“‘±ΫΈΣ‘≠Νœ…ζ≥…![]() ΒΡΜ·―ßΖΫ≥Χ Ϋ «
ΒΡΜ·―ßΖΫ≥Χ Ϋ «![]() +Br2
+Br2 ![]() +HBrΓΘ
+HBrΓΘ
¥πΑΗΈΣΘΚ![]() +Br2
+Br2 ![]() +HBrΘΜ
+HBrΘΜ
Θ®3Θ©![]() ”κNaOH»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ
”κNaOH»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ Ϋ![]() +2NaOH
+2NaOH ![]() +NaBr+ H2OΓΘ¥πΑΗΈΣΘΚ
+NaBr+ H2OΓΘ¥πΑΗΈΣΘΚ![]() +2NaOH
+2NaOH ![]() +NaBr+ H2OΘΜ
+NaBr+ H2OΘΜ
Θ®4Θ©”… ÷Τ±Η
÷Τ±Η ![]() ΒΡΖ¥”ΠΧθΦΰ «≈®ΝρΥαΓΔΦ”»»ΓΘ
ΒΡΖ¥”ΠΧθΦΰ «≈®ΝρΥαΓΔΦ”»»ΓΘ
¥πΑΗΈΣΘΚ≈®ΝρΥαΓΔΦ”»»ΘΜ
Θ®5Θ©ΖϊΚœœ¬Ν–ΧθΦΰΒΡ ΒΡΆ§Ζ÷“λΙΙΧεΓΘΔΌΖ÷Ή”÷–≥ΐ±ΫΜΖΆβ≤ΜΚ§ΤδΥϊΜΖΉ¥ΫαΙΙΘΜΔΎ±ΫΜΖ…œ÷Μ”–2Ηω»Γ¥ζΜυΘ§«“Τδ÷–“ΜΗω «»©ΜυΓΘ
ΒΡΆ§Ζ÷“λΙΙΧεΓΘΔΌΖ÷Ή”÷–≥ΐ±ΫΜΖΆβ≤ΜΚ§ΤδΥϊΜΖΉ¥ΫαΙΙΘΜΔΎ±ΫΜΖ…œ÷Μ”–2Ηω»Γ¥ζΜυΘ§«“Τδ÷–“ΜΗω «»©ΜυΓΘ
‘ρΝμ“Μ»Γ¥ζΜυΩ…“‘ «œ¬Ν–«ιΩωœ¬ΒΡΡ≥“Μ÷÷ΘΚ-CH2CH2CH=CH2ΓΔ-CH2CH=CHCH2ΓΔ
-CH=CHCH2CH3ΓΔ-CH2C(CH3)=CH2ΓΔ-CH(CH3)CH=CH2ΓΔ-CH=C(CH3)2ΓΔ-C(CH3)=CHCH3ΓΔ
-C(CH2CH3)=CH2Θ§ΟΩΗω»Γ¥ζΜυ”κ»©Μυ”÷Ω…ΈΜ”ΎΝΎΓΔΦδΓΔΕ‘ΒΡΈΜ÷ΟΘ§Υυ“‘“λΙΙΧεΒΡ ΐΡΩΙ≤”–ΘΚ
3ΓΝ8=24ΓΘ¥πΑΗΈΣΘΚ24ΓΘ

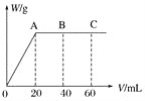
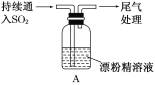
ΓΨΧβΡΩΓΩΡ≥―ß…ζΕ‘SO2”κΤ·ΖέΨΪΒΡΖ¥”ΠΫχ–– Β―ιΧΫΨΩΘΚ
≤ΌΉς | œ÷œσ |
»Γ4gΤ·ΖέΨΪΙΧΧεΘ§Φ”»κ100mLΥ° | ≤ΩΖ÷ΙΧΧε»ήΫβΘ§»ή“Κ¬‘”–―’…Ϊ |
Ιΐ¬ΥΘ§≤βΤ·ΖέΨΪ»ή“ΚΒΡpH | pH ‘÷Ϋœ»±δάΕ(‘ΦΈΣ12)Θ§ΚσΆ …Ϊ |
| i.“ΚΟφ…œΖΫ≥ωœ÷ΑΉΈμΘΜ ii.…‘ΚσΘ§≥ωœ÷ΜκΉ«Θ§»ή“Κ±δΈΣΜΤ¬Χ…ΪΘΜ iii.…‘ΚσΘ§≤ζ…ζ¥σΝΩΑΉ…Ϊ≥ΝΒμΘ§ΜΤ¬Χ…ΪΆ »Ξ |
Θ®1Θ©Cl2ΚΆCa(OH)2÷Τ»ΓΤ·ΖέΨΪΒΡΜ·―ßΖΫ≥Χ Ϋ «___ΓΘ
Θ®2Θ©pH ‘÷Ϋ―’…ΪΒΡ±δΜ·ΥΒΟςΤ·ΖέΨΪ»ή“ΚΨΏ”–ΒΡ–‘÷ «___ΓΔ___ΓΘ
Θ®3Θ©œρΥ°÷–≥÷–χΆ®»κSO2Θ§Έ¥Ιέ≤λΒΫΑΉΈμΓΘΆΤ≤βœ÷œσiΒΡΑΉΈμ”…HCl–Γ“ΚΒΈ–Έ≥…ΓΘΫχ––»γœ¬ Β―ιΘΚ
aΘ°”Ο Σ»σΒΡΒβΜ·ΦΊΒμΖέ ‘÷ΫΦλ―ιΑΉΈμΘ§Έό±δΜ·ΘΜ
bΘ°”ΟΥαΜ·ΒΡAgNO3»ή“ΚΦλ―ιΑΉΈμΘ§≤ζ…ζΑΉ…Ϊ≥ΝΒμΓΘ
ΔΌ Β―ιaΒΡΡΩΒΡ «___ΓΘ
ΔΎ”… Β―ιaΓΔb≤ΜΡή≈–ΕœΑΉΈμ÷–Κ§”–HClΘ§άμ”… «___ΓΘ
Θ®4Θ©ΫΪAΤΩ÷–ΜλΚœΈοΙΐ¬ΥΓΔœ¥Β”Θ§ΒΟΒΫ≥ΝΒμX
ΔΌœρ≥ΝΒμX÷–Φ”»κœΓHClΘ§ΈόΟςœ‘±δΜ·ΓΘ»Γ…œ≤ψ«ε“ΚΘ§Φ”»κBaCl2»ή“ΚΘ§≤ζ…ζΑΉ…Ϊ≥ΝΒμΓΘ‘ρ≥ΝΒμX÷–Κ§”–ΒΡΈο÷ «___ΓΘ
ΔΎ”ΟάκΉ”ΖΫ≥Χ ΫΫβ Άœ÷œσiii÷–ΜΤ¬Χ…ΪΆ »ΞΒΡ‘≠“ρΘΚ___ΓΘ
Θ®5Θ©≤βΕ®Τ·ΖέΨΪ”––ß≥…Ζ÷ΒΡ÷ ΝΩΖ÷ ΐΓΘ≥Τ»Γ2.000gΤ·ΖέΨΪ”ΎΉΕ–ΈΤΩ÷–Θ§Φ”Υ°»ήΫβΘ§ΒςΫΎ»ή“ΚΒΡpHΘ§“‘ΒμΖέΈΣ÷Η ΨΦΝΘ§”Ο0.2000molΓΛL-1 KI»ή“ΚΫχ––ΒΈΕ®Θ§»ή“Κ≥ωœ÷Έ»Ε®«≥άΕ…Ϊ ±ΈΣΒΈΕ®÷’ΒψΓΘ
Ζ¥”Π‘≠άμΈΣΘΚ3C1O-+I-=3C1-+IO3-Θ§IO3- +5I-+3H2O=6OH-+3I2
Β―ι≤βΒΟ ΐΨί»γœ¬±μΥυ ΨΓΘ
ΒΈΕ®¥Έ ΐ | 1 | 2 | 3 |
KI»ή“ΚΧεΜΐ/mL | 19.98 | 20.02 | 20.00 |
ΗΟΤ·ΑΉΖέ÷–”––ß≥…Ζ÷ΒΡ÷ ΝΩΖ÷ ΐΈΣ___ΓΘ