��Ŀ����
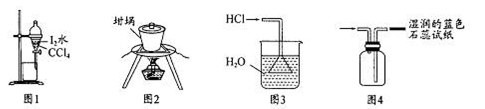
�ײ��к��и�����Ԫ�أ�ij��ѧС��������·����ⶨ�ɰײ��и�Ԫ�ص���������������ȡ10.00g�ɰײ�Ҷ�����յðײ˻ҷ۽�������ʵ�飺
��1��ʵ��ǰҪ�Ƚ��ɰײ�Ҷ��Ʒ�������ճɻҷۣ�����ҪĿ����ʹ��Ʒ�е��л�����ȫ�ֽ⣬ʹ�ɰײ�Ҷ�еĸơ���Ԫ���ܽ���ȫ�������õ��IJ���������
A������ B�������� C�������� D��������
��2��д������ҺA������D�����ӷ�Ӧ����ʽ ��
��3����KMnO4����Һ�ζ���ҺC���Ƚ���ҺCϡ����500 mL����ȡ���е�25��00 mL��Һ���������ữ����0.100 0 mol��L-1����KMnO4����Һ�ζ����յ�ʱ����KMnO4��Һ10.00mL��
�����ķ�ӦΪ��
�ٵζ��Ĺ����У�ͬѧ�Ƿ���һ��������C��Һ�м����һ��KMnO4��Һʱ����Ҫ��ҡ��ƿ�ϳ�ʱ�������ɫ������Һ��ɫ���ٵ���KMnO4��Һ����Ѹ����ɫ��ֱ���ﵽ�յ㣻Ϊ�˼ӿ�����һ��KMnO4��Һʱ����ɫ�ٶȣ��ɲ�ȡ�ķ����� .��ѡ����ʵ�ѡ�
A���ʵ�������ƿ����Һ B������ƿ�ڼ�����ˮ
C������ƿ�ڼ������Ҵ� D������ƿ�ڼ��뼸��MnSO4��Һ
���жϵζ��ﵽ�յ�ķ����� ��
��4��ԭ�ɰײ�Ҷ�и�Ԫ�ص���������Ϊ ��
��5��Ϊ��֤ʵ�龫ȷ�ȣ�����D��E��Ҫ�ֱ�ϴ�ӣ�����ϴ��Һת�ƻ�ĸҺ�У����жϳ���D�Ѿ�ϴ�Ӹɾ��ķ����� ���������Eδϴ�ӣ���δ��ϴ��Һת�ƻ�ĸҺ�����õĸ�Ԫ���������� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����

��1��ʵ��ǰҪ�Ƚ��ɰײ�Ҷ��Ʒ�������ճɻҷۣ�����ҪĿ����ʹ��Ʒ�е��л�����ȫ�ֽ⣬ʹ�ɰײ�Ҷ�еĸơ���Ԫ���ܽ���ȫ�������õ��IJ���������
A������ B�������� C�������� D��������
��2��д������ҺA������D�����ӷ�Ӧ����ʽ ��
��3����KMnO4����Һ�ζ���ҺC���Ƚ���ҺCϡ����500 mL����ȡ���е�25��00 mL��Һ���������ữ����0.100 0 mol��L-1����KMnO4����Һ�ζ����յ�ʱ����KMnO4��Һ10.00mL��
�����ķ�ӦΪ��

�ٵζ��Ĺ����У�ͬѧ�Ƿ���һ��������C��Һ�м����һ��KMnO4��Һʱ����Ҫ��ҡ��ƿ�ϳ�ʱ�������ɫ������Һ��ɫ���ٵ���KMnO4��Һ����Ѹ����ɫ��ֱ���ﵽ�յ㣻Ϊ�˼ӿ�����һ��KMnO4��Һʱ����ɫ�ٶȣ��ɲ�ȡ�ķ����� .��ѡ����ʵ�ѡ�
A���ʵ�������ƿ����Һ B������ƿ�ڼ�����ˮ
C������ƿ�ڼ������Ҵ� D������ƿ�ڼ��뼸��MnSO4��Һ
���жϵζ��ﵽ�յ�ķ����� ��
��4��ԭ�ɰײ�Ҷ�и�Ԫ�ص���������Ϊ ��
��5��Ϊ��֤ʵ�龫ȷ�ȣ�����D��E��Ҫ�ֱ�ϴ�ӣ�����ϴ��Һת�ƻ�ĸҺ�У����жϳ���D�Ѿ�ϴ�Ӹɾ��ķ����� ���������Eδϴ�ӣ���δ��ϴ��Һת�ƻ�ĸҺ�����õĸ�Ԫ���������� ���ƫ�ߡ���ƫ�͡�����Ӱ�족����
����13�֣���ѧ��Ӧʽ��ʽδ��ƽ�ľ���1��
��1�� ACD��2�֣���ѡ1����1�֣��д�ѡ���÷֣�
��2�� Fe3����3NH3��H2O��Fe(OH)3����3NH4+��2�֣���NH3��H2O��д�ɡ�NH3��H2O�����۷֣��ޡ�������1�֣�д�ɻ�ѧ����ʽ���÷֣�
��3���� AD ��2�֣���ѡ1����1�֣��д�ѡ���÷֣�
�� �������һ��KMnO4��Һ����Һ�Ժ�ɫ�Ұ��������Һ����ɫ����1�֣��ᵽ����Һ����ɫ������ɫ�����Ϻ�ɫ����ɫ�����ɵ÷֣�
��4��4.000%��3�֣���Ч���ֲ����ǣ�
��5��ȡ���һ��ϴ��Һ�������Թ��У��μ�Na2CO3��Һ������������������ϴ�Ӹɾ�����2�֣��Լ�1�֣�����1�֣�������������Ҳ���֣�
ƫ�ߣ�1�֣�
��1�� ACD��2�֣���ѡ1����1�֣��д�ѡ���÷֣�
��2�� Fe3����3NH3��H2O��Fe(OH)3����3NH4+��2�֣���NH3��H2O��д�ɡ�NH3��H2O�����۷֣��ޡ�������1�֣�д�ɻ�ѧ����ʽ���÷֣�
��3���� AD ��2�֣���ѡ1����1�֣��д�ѡ���÷֣�
�� �������һ��KMnO4��Һ����Һ�Ժ�ɫ�Ұ��������Һ����ɫ����1�֣��ᵽ����Һ����ɫ������ɫ�����Ϻ�ɫ����ɫ�����ɵ÷֣�
��4��4.000%��3�֣���Ч���ֲ����ǣ�
��5��ȡ���һ��ϴ��Һ�������Թ��У��μ�Na2CO3��Һ������������������ϴ�Ӹɾ�����2�֣��Լ�1�֣�����1�֣�������������Ҳ���֣�
ƫ�ߣ�1�֣�
�������������ҪŪ���������Ԫ�����������ⶨ�Ĺ������̡����ײ˾���һϵ�еIJ������õ���ҺA����ҺA�к��е�������Fe3����Ca2+�����백ˮ����pH=6��Ŀ���ǽ�Fe3����ȥ��Ȼ��õ���ҺB�ͳ���D Fe(OH)3������ҺB�м������泥�Ŀ���ǽ�Ca2+������ɲ���ƣ�����ɫ����E��ͨ���ⶨ��ɫ����E���������ⶨ�ײ��и�Ԫ�ص�����������
���ոɰײ�Ҷ����Ҫ��������Ҫ�У����������������ƾ��ƣ������ǡ��������ᾧ��������������
����ҺA������D�����ӷ�Ӧ����ʽΪ��Fe3����3NH3��H2O��Fe(OH)3����3NH4+
��ҺC���й����IJ���泥�ͨ���ø��������Һ���ⶨ�����IJ���泥��Ӷ����Եó���Ca2+��Ӧ�IJ���泥��Ӷ�����������ӵĺ�����
��Ϊ�˼ӿ�����һ��KMnO4��Һʱ����ɫ�ٶȣ����ӿ췴Ӧ�ٶȣ������ʵ��ļ�����ƿ�ڵ���Һ��Ҳ���Լ��뼸��MnSO4��Һ����ΪMn2+�д��÷�Ӧ�ٶȵ����á�
�ڵ���Ӧ�ﵽ�ζ��յ�ʱ��Ҳ�������һ�θ��������Һ���룬������Ѿ��������ˣ���ʱ��Һ����ɫ�����ɫ������30s�ڲ���ɫ�����ζ��ﵽ�յ㡣
ԭ�ɰײ�Ҷ�и�Ԫ�ص����������IJⶨ��
��Һ�й�����n��C2O42-��=
 n��MnO4-��=
n��MnO4-��= ��0.1��0.01="0.0025" mol
��0.1��0.01="0.0025" mol����Ca2+��Ӧ�IJ����n��C2O42-��=
 =0.01mol
=0.01mol����n��Ca2+��= n��C2O42-��=0.01mol
w��Ca��=
 =4.000%
=4.000%��5���жϳ����Ƿ�ϴ������Ҫ���������Ƿ��п����Ե����ӣ�����ȡ���һ��ϴ�ӵ���Һ�������Һ���Ƿ���Ca2+ ���ɡ����������ȡ���һ��ϴ��Һ�������Թ��У��μ�Na2CO3��Һ������������������ϴ�Ӹɾ�������EΪ����ƣ������Ͽ��ܸ��п����������磺C2O42-����δϴ�ӣ�������ø��������Һ�ζ���Һ�е�C2O42-�������ĵĸ�����ص���ƫ�٣���ô������ӷ�Ӧ�IJ�������ӵ�����ƫ�ߣ����ղ�õİײ�Ҷ�еĸ����ӵĺ���ƫ�ߡ�

��ϰ��ϵ�д�
�����Ŀ
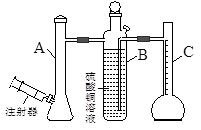
 +I2= 2I��+S4O
+I2= 2I��+S4O