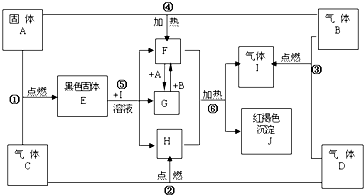
��Ŀ����
����A��B��C��D��E��F��G��H��I��J��K������ͼת����ϵ����������D��EΪ���ʣ��Իش�
��1��AԪ�ص�ԭ�ӽṹʾ��ͼΪ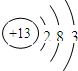
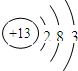 ��
��
��2��д���������ʵĻ�ѧʽ��E��
��3��������B����
��4��ʵ���Ҽ���J�������Ľ�������ʱ������J����Һ�м���
��5��д����Ӧ��F��G���Ļ�ѧ����ʽ��
��6��д����Ӧ��I��J�������ӷ���ʽ��

��1��AԪ�ص�ԭ�ӽṹʾ��ͼΪ
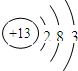
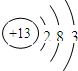
��2��д���������ʵĻ�ѧʽ��E��
Cl2
Cl2
��K��Fe��OH��3
Fe��OH��3
����3��������B����
����
����
���������ӡ����ۡ�������4��ʵ���Ҽ���J�������Ľ�������ʱ������J����Һ�м���
���軯����Һ������������Һ
���軯����Һ������������Һ
��Һ����5��д����Ӧ��F��G���Ļ�ѧ����ʽ��
Al��OH��3+NaOH=NaAlO2+2H2O
Al��OH��3+NaOH=NaAlO2+2H2O
����6��д����Ӧ��I��J�������ӷ���ʽ��
2Fe2++Cl2=2Fe2++2Cl-
2Fe2++Cl2=2Fe2++2Cl-
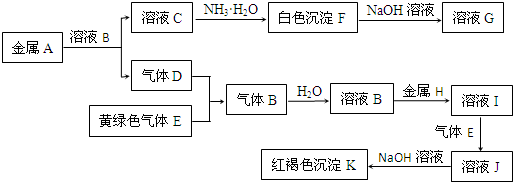
��������KΪ���ɫ��������ӦΪFe��OH��3������ҺJ�к���Fe3+������HΪFe��DӦΪH2��EӦΪCl2��BΪHCl����IΪFeCl2��JΪFeCl3��KΪFe��OH��3����ɫ����F�����ڹ���NaOH��Һ����FΪAl��OH��3��GΪNaAlO2��AΪAl��CΪAlCl3���������ʵ������ж��������ķ�Ӧ���������⣮
����⣺KΪ���ɫ��������ӦΪFe��OH��3������ҺJ�к���Fe3+������HΪFe��DӦΪH2��EӦΪCl2��BΪHCl����IΪFeCl2��JΪFeCl3��KΪFe��OH��3����ɫ����F�����ڹ���NaOH��Һ����FΪAl��OH��3��GΪNaAlO2��AΪAl��CΪAlCl3��
��1��AΪAl��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ3����ԭ�ӽṹʾ��ͼΪ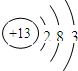 ��
��
�ʴ�Ϊ��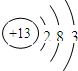 ��
��
��2�������Ϸ�����֪EΪCl2��KΪFe��OH��3���ʴ�Ϊ��Cl2��Fe��OH��3��
��3��BΪHCl��Ϊ���ۻ�����ʴ�Ϊ�����ۣ�
��4��JΪFeCl3���������軯�ط�����ɫ��Ӧ����Һ�ʺ�ɫ����������������Һ�����������������ɫ������
�ʴ�Ϊ�����軯����Һ������������Һ��
��5��FΪAl��OH��3��GΪNaAlO2����Ӧ��F��G���Ļ�ѧ����ʽΪAl��OH��3+NaOH=NaAlO2+2H2O��
�ʴ�Ϊ��Al��OH��3+NaOH=NaAlO2+2H2O��
��6��IΪFeCl2��JΪFeCl3����Ӧ��I��J�������ӷ���ʽΪ2Fe2++Cl2=2Fe2++2Cl-��
�ʴ�Ϊ��2Fe2++Cl2=2Fe2++2Cl-��
��1��AΪAl��ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ3����ԭ�ӽṹʾ��ͼΪ
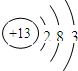 ��
���ʴ�Ϊ��
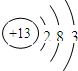 ��
����2�������Ϸ�����֪EΪCl2��KΪFe��OH��3���ʴ�Ϊ��Cl2��Fe��OH��3��
��3��BΪHCl��Ϊ���ۻ�����ʴ�Ϊ�����ۣ�
��4��JΪFeCl3���������軯�ط�����ɫ��Ӧ����Һ�ʺ�ɫ����������������Һ�����������������ɫ������
�ʴ�Ϊ�����軯����Һ������������Һ��
��5��FΪAl��OH��3��GΪNaAlO2����Ӧ��F��G���Ļ�ѧ����ʽΪAl��OH��3+NaOH=NaAlO2+2H2O��
�ʴ�Ϊ��Al��OH��3+NaOH=NaAlO2+2H2O��
��6��IΪFeCl2��JΪFeCl3����Ӧ��I��J�������ӷ���ʽΪ2Fe2++Cl2=2Fe2++2Cl-��
�ʴ�Ϊ��2Fe2++Cl2=2Fe2++2Cl-��
���������⿼��������ƶϣ���Ŀ�Ѷ��еȣ�����ע��������ʵ��������Խ�����ʵ�������Ϊͻ�ƿڽ����ƶϣ�ѧϰ��ע�����������ʵ����ʣ�

��ϰ��ϵ�д�
�����Ŀ