��Ŀ����
�������ֿ���������A��B��C��D��E���������������������ӻ�����ͬ���ֱ��� ����������Na+��Al3+��Mg2+��Ba2+��Fe3+������������Cl-��OH-��NO
��CO
��X�� ��һ�֣�
��1��ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е����������� �� ���ѧʽ����
��2��Ϊ��ȷ��X���ֽ���1���е��������ʼ�ΪA��B������X���ӵ�C��A����Һ�� ��ʱ���������ɫ��������ɫ��ζ���壻��C��B����Һ���ʱ�������ֳ�������ó����е���������ϡHNO3�����������ܽ⣬������а�ɫ��������
��XΪ ������ţ���
A��SiO
B��CH3COO-C��SO
��A��ˮ��Һ���������ӵ�Ũ���ɴ�С��˳��Ϊ ��
�ۻ�����B�ĵ���ʽ ��
�ܽ�0.02mol��B��0.01mol��Cͬʱ�ܽ�������������ˮ�У���ַ�Ӧ������ ���ó���������Ϊ g����ȷ��0.1g����
�����������Ѿ�ȷ����һ�����ʣ����Լ����D��E�е������ӣ������ʵ������� �衢������ ��
��3����CuƬͶ�뵽װ��D��Һ���Թ��У�CuƬ���ܽ⣻�ٵμ�ϡH2SO4��CuƬ�� �ܽ⣬�Թܿڸ����к���ɫ������֣��йط�Ӧ�����ӷ���ʽΪ ��
- 3 |
2- 3 |
��1��ijͬѧͨ���ȽϷ�������Ϊ�������Ϳ��ж����б��е�����������
��2��Ϊ��ȷ��X���ֽ���1���е��������ʼ�ΪA��B������X���ӵ�C��A����Һ�� ��ʱ���������ɫ��������ɫ��ζ���壻��C��B����Һ���ʱ�������ֳ�������ó����е���������ϡHNO3�����������ܽ⣬������а�ɫ��������
��XΪ
A��SiO
2- 3 |
2- 4 |
��A��ˮ��Һ���������ӵ�Ũ���ɴ�С��˳��Ϊ
�ۻ�����B�ĵ���ʽ
�ܽ�0.02mol��B��0.01mol��Cͬʱ�ܽ�������������ˮ�У���ַ�Ӧ������ ���ó���������Ϊ
�����������Ѿ�ȷ����һ�����ʣ����Լ����D��E�е������ӣ������ʵ������� �衢������
��3����CuƬͶ�뵽װ��D��Һ���Թ��У�CuƬ���ܽ⣻�ٵμ�ϡH2SO4��CuƬ�� �ܽ⣬�Թܿڸ����к���ɫ������֣��йط�Ӧ�����ӷ���ʽΪ
��������1������̼������Ӻͱ����������������ܹ��γɵĿ��������ʷ�����
��2���ٸ��ݵ���X���ӵ�C��A����Һ�� ��ʱ���������ɫ��������ɫ��ζ���壬˵��������˫ˮ�⣬�ƶϳ�AΪ̼���ƣ���BΪ�����������ٸ��ݵ�C��B����Һ���ʱ�������ֳ�������ó����е���������ϡHNO3�����������ܽ⣬������а�ɫ�������ƶϳ�CΪ��������
�ڸ���̼������Һ��ˮ����ʾ���ԣ�д����Һ������Ũ�ȴ�С��ϵ��
�۸������ӻ�����ĵ���ʽ��д����д�����������ĵ���ʽ��
�ܸ��������������������б����ӡ���������ӡ������Ӻ����������ӵ����ʵ����жϹ��������ݲ��������������ɵij���������
����������������Һ����̼������Һ���м���
��3����CuƬͶ�뵽װ��D��Һ���Թ��У�CuƬ���ܽ⣻�ٵμ�ϡH2SO4��CuƬ���ܽ⣬֤��D�к�����������ӣ�д��ͭ��ϡ���ᷴӦ�����ӷ���ʽ��
��2���ٸ��ݵ���X���ӵ�C��A����Һ�� ��ʱ���������ɫ��������ɫ��ζ���壬˵��������˫ˮ�⣬�ƶϳ�AΪ̼���ƣ���BΪ�����������ٸ��ݵ�C��B����Һ���ʱ�������ֳ�������ó����е���������ϡHNO3�����������ܽ⣬������а�ɫ�������ƶϳ�CΪ��������
�ڸ���̼������Һ��ˮ����ʾ���ԣ�д����Һ������Ũ�ȴ�С��ϵ��
�۸������ӻ�����ĵ���ʽ��д����д�����������ĵ���ʽ��
�ܸ��������������������б����ӡ���������ӡ������Ӻ����������ӵ����ʵ����жϹ��������ݲ��������������ɵij���������
����������������Һ����̼������Һ���м���
��3����CuƬͶ�뵽װ��D��Һ���Թ��У�CuƬ���ܽ⣻�ٵμ�ϡH2SO4��CuƬ���ܽ⣬֤��D�к�����������ӣ�д��ͭ��ϡ���ᷴӦ�����ӷ���ʽ��
����⣺��1������������Al3+��Mg2+��Ba2+��Fe3+��������CO32-���棬����CO32-ֻ����Na+��ϳ�Na2CO3���ڣ�����Al3+��Mg2+��Fe3+��������OH-���棬����OH-ֻ����Ba2+��ϳ�Ba��OH��2���ڣ�
�ʴ�Ϊ��Na2CO3��Ba��OH��2��
��2���ٲ������ɫ��������ɫ��ζ���壬��������Fe3+��CO32-����˫ˮ��������ѵó�AΪNa2CO3��BΪBa��OH��2��C����Fe3+������C��A��Ӧ�����İ�ɫ����������ϡHNO3����CΪFe2��SO4��3������XΪSO42-��
��ѡC��
�ڸ��ݢٿ�֪��AΪ̼������Һ��̼������ӷ�����ˮ�⣬��Һ��ʾ���ԣ�Na2CO3��Һ�и�����Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ۻ�����BΪ������������������Ϊ���ӻ�������ӻ�����ĵ���ʽ��Ҫ��������ĵ�ɣ����������ĵ���ʽΪ��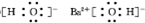 ��
��
�ʴ�Ϊ��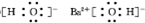 ��
��
��0.02mol��Ba��OH��2��0.01mol��Fe2��SO4��3��Ӧ����0.02mol Ba2+��0.04mol OH-��0.02mol Fe3+��0.03molSO42-��������0.02molBaSO4��0.04/3mol Fe��OH��3����������ó�������Ϊ��0.02mol��233g/mol+0.04mol3��107g/mol��6.1g��
�ʴ�Ϊ��6.1��
���������Ѿ�ȷ��������������Һ����̼������Һ�����Լ����D��E�е������ӣ����鷽��Ϊ����D����Һ������Ba��OH��2��Һֱ�����������ȳ��ְ�ɫ�����������ܽ⣬��D�к���Al3+��E�к���Mg2+��������D����Һ�м�������Na2CO3��Һ�������˰�ɫ��������ɫ��ζ�����壬��D�к���Al3+��E�к���Mg2+����
�ʴ�Ϊ����D����Һ������Ba��OH��2��Һֱ�����������ȳ��ְ�ɫ�����������ܽ⣬��D�к���Al3+��E�к���Mg2+��������D����Һ�м�������Na2CO3��Һ�������˰�ɫ��������ɫ��ζ�����壬��D�к���Al3+��E�к���Mg2+����
��3��ͭ������ϡ���ᣬ��������ϡ���ᣬ����D��һ������NO3-����Ӧ�����ӷ���ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
�ʴ�Ϊ��Na2CO3��Ba��OH��2��
��2���ٲ������ɫ��������ɫ��ζ���壬��������Fe3+��CO32-����˫ˮ��������ѵó�AΪNa2CO3��BΪBa��OH��2��C����Fe3+������C��A��Ӧ�����İ�ɫ����������ϡHNO3����CΪFe2��SO4��3������XΪSO42-��
��ѡC��
�ڸ��ݢٿ�֪��AΪ̼������Һ��̼������ӷ�����ˮ�⣬��Һ��ʾ���ԣ�Na2CO3��Һ�и�����Ũ�ȴ�СΪ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ʴ�Ϊ��c��Na+����c��CO32-����c��OH-����c��HCO3-����c��H+����
�ۻ�����BΪ������������������Ϊ���ӻ�������ӻ�����ĵ���ʽ��Ҫ��������ĵ�ɣ����������ĵ���ʽΪ��
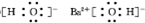 ��
���ʴ�Ϊ��
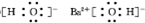 ��
����0.02mol��Ba��OH��2��0.01mol��Fe2��SO4��3��Ӧ����0.02mol Ba2+��0.04mol OH-��0.02mol Fe3+��0.03molSO42-��������0.02molBaSO4��0.04/3mol Fe��OH��3����������ó�������Ϊ��0.02mol��233g/mol+0.04mol3��107g/mol��6.1g��
�ʴ�Ϊ��6.1��
���������Ѿ�ȷ��������������Һ����̼������Һ�����Լ����D��E�е������ӣ����鷽��Ϊ����D����Һ������Ba��OH��2��Һֱ�����������ȳ��ְ�ɫ�����������ܽ⣬��D�к���Al3+��E�к���Mg2+��������D����Һ�м�������Na2CO3��Һ�������˰�ɫ��������ɫ��ζ�����壬��D�к���Al3+��E�к���Mg2+����
�ʴ�Ϊ����D����Һ������Ba��OH��2��Һֱ�����������ȳ��ְ�ɫ�����������ܽ⣬��D�к���Al3+��E�к���Mg2+��������D����Һ�м�������Na2CO3��Һ�������˰�ɫ��������ɫ��ζ�����壬��D�к���Al3+��E�к���Mg2+����
��3��ͭ������ϡ���ᣬ��������ϡ���ᣬ����D��һ������NO3-����Ӧ�����ӷ���ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
�ʴ�Ϊ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��
���������⿼���˳������ӵļ��鷽������Ŀ�ѶȽϴ���ѧ�����ۺ�����֪ʶ�����������ü��跨���ų�������ɸѡ�������жϳ��������ӵ���ϣ��ڼ����ʱ��Ӧע���������⣬�ҳ����������ӣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ