��Ŀ����
��ɰֽ�����þ��ؤ��ˮ��Ӧ������Ӧ����Һ�еμӷ�̪��Һ�����Ƶ�H2S������Һ�еμ����Ƶ���ˮ��������з�̪����ˮ��Ӧþ����2mol��L-1���ᷴӦ������2mol��L-1���ᷴӦ��ALCI3��Һ�еμ�NaOH��Һ������A. ����ˮ�棬�۳�������С���ı��ζ�������С����Һ���ɫ
B. �������������壬�����ȼ����Һ��dz��ɫ
C. ��Ӧ���־��ң������������ȼ
D. ���ҷ�Ӧ�������������ȼ
E. ���ɰ�ɫ��״�������̶�������ʧ
F. ���ɵ���ɫ����
��3��ʵ�����ݣ�
ʵ�鷽���������������������������������� ʵ�������������������������������������� �йػ�ѧ����
�������������������������������������������� ��������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��������������������������������������������������������������������������������������������
��4��ʵ����ۣ�________��
��5���������ۣ�����ӽṹ�����ϼ�˵���������۵�ԭ��
�����㲹��һ��ʵ�鷽���������У���֤��������������������Ԫ�ص����ʵݱ���ɡ�
�������ж�����Ԫ�����ʵ����ݣ�
Ԫ�ر��
Ԫ�������������� ���������� ���������� ���������� ���������� ���������� ���������� ���������� ��
ԭ�Ӱ뾶
��10-10m������ 0.74�������� 1.60�������� 1.52�������� 1.10�������� 0.99�������� 1.86�������� 0.75�������� 0.82
��ߺ���
�ͻ��ϼ������������������������� +2�������� +1�������� +5�������� +7�������� +1�������� +5�������� +3
�������������������� -2���������������������������������������� -3�������� -1������������������������ -3��������
�Իش��������⣺
��1������Ԫ���д���ͬһ�������________��Ԫ�آ������ڱ��е�λ��Ϊ________��
��2���ϱ���ij����Ԫ�أ��γɵķ����У�ÿ��ԭ�Ӷ����������Ϊ8���ӵ��ȶ��ṹ��д�������ʽ________��
��3��Ԫ�آ٢����γ����ֻ����д�����н��ȶ��Ļ���������ˮ��Ӧ�ģ����ӷ���ʽ��________��
������
| ��1����֤��������Ԫ�ش����ҽ����Ժͷǽ����Եĵݱ���ɡ�
��2���������Թܡ��ձ����ƾ��ơ���ֽ��ɰֽ�����ӣ�С������ͷ�ιܡ���� �Լ����ơ�þ������Ƭ��������ˮ�����Ʊ���H2S��Һ2mol��L��1���ᡢNaOH��Һ������ˮ����̪��Һ��AlCl3��Һ�� ��3��1 B Mg��2H2O=Mg��OH��2��H2�� 2 F H2S��Cl2=2HCl��S�� 3 A 2Na��2H2O=2NaOH��H2�� 4 D Mg��2HCl=MgCl2��H2�� 5 C 2Al��6HCl=2AlCl3��3H2�� 6 E AlCl3��3NaOH=Al��OH��3����3NaCl Al��OH��3��NaOH=NaAlO2��2H2O ��4��Na��Mg��Al��S��ClԪ�أ�����ԭ�������ĵ�����Ԫ�صĽ������������ǽ���������ǿ�� ��5����ͬһ����Ԫ�ش���������ԭ�������ĵ�����ԭ�Ӱ뾶��С���˶������ӵ�����������ǿ�����ʧ���ӵ������������õ��ӵ���������ǿ��Ԫ�صĽ������������ǽ���������ǿ�� ��������δ��֤��Ԫ��ΪSi��P�������������ʵ��ȷ����ǽ�����ǿ������Na2SiO3��Һ�еμ�H3PO4��Һ����������ɫ��״��������˵��H3PO4��H2SiO3����ǿ���Ӷ�֤����Ԫ�صķǽ����Աȹ�ǿ����������������Ҳ�ɣ�
|

 ��ʦ�㾦�ִʾ��ƪϵ�д�
��ʦ�㾦�ִʾ��ƪϵ�д�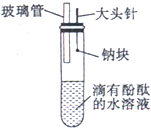 ijͬѧ��ͬ����Ԫ�����ʵݱ����ʵ��ʱ���Լ������һ��ʵ�鷽��������¼���й�ʵ���������±���
ijͬѧ��ͬ����Ԫ�����ʵݱ����ʵ��ʱ���Լ������һ��ʵ�鷽��������¼���й�ʵ���������±���