��Ŀ����
�绯ѧԭ���ڹ�ҵ������������Ҫ�����ã���������ѧ֪ʶ�ش��й����⡣
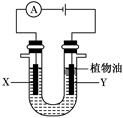
(1)�õ��ķ�����������Һ����Ϊ��������о�������Ҫ��ʵ�����壬������ת��Ϊ�������ǵ�ⷨ�������������һ����Ҫ���ݡ����ǵ������������ʵ��װ�ã�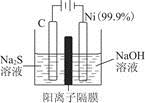
����֪�����ķ�ӦΪ(x��1)S2��=Sx��S2����2xe�����������ĵ缫��Ӧʽ��____________________________
����Ӧת��x mol����ʱ���������������Ϊ____________(��״����)��
�ڽ�Na2S��9H2O����ˮ������������Һʱ��ͨ�����ڵ����������ܽ⡣��ԭ����(�����ӷ�Ӧ����ʽ��ʾ)��___________________________��
(2)MnO2��һ����Ҫ�������ܲ��ϣ��Ʊ�MnO2�ķ���֮һ����ʯīΪ�缫������ữ��MnSO4��Һ�������ĵ缫��ӦʽΪ______________________������Ǧ����Ϊ��Դ����ữ��MnSO4��Һ����ͼ��ʾ��Ǧ���ص��ܷ�Ӧ����ʽΪ_______________________��
����������4 mol H��������ʱ�����·��ͨ���ĵ��ӵ����ʵ���Ϊ________��MnO2�����۲���Ϊ________g��
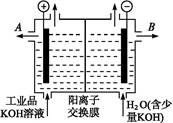
(3)��ͼ���װ�ÿ��Ƶþ��о�ˮ���õ� ��ʵ������У������������������Y������Һ������
��ʵ������У������������������Y������Һ������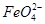

�ٵ������У�X������Һ��pH________(�������С�����䡱)��
�ڵ������У�Y�������ĵ缫��ӦΪFe��6e����8OH��=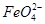 ��4H2O��______________________________������X���ռ���672 mL���壬��Y���ռ���168 mL����(��������Ϊ��״��ʱ�������)����Y�缫(���缫)��������________g��
��4H2O��______________________________������X���ռ���672 mL���壬��Y���ռ���168 mL����(��������Ϊ��״��ʱ�������)����Y�缫(���缫)��������________g��
(1)��2H2O��2e��=2OH����H2��(��2H����2e��=H2��)��11.2xL
��2S2����O2��2H2O=2S����4OH��
(2)Mn2����2e����2H2O=MnO2��4H��
Pb��PbO2��2H2SO4=2PbSO4��2H2O
2 mol��87
(3)����4OH����4e��=2H2O��O2����0.28
����(1)���ʱ��ˮ�����H�������������õ��ӻ�ԭ��Ӧ������H2�����ݵ����غ��֪��x mol����ת�ƣ�����H2 0.5x mol��S2�����н�ǿ��ԭ�ԣ��ױ������е�������������������Һʱ��Ҫ��������������
(2)��������Mn2��ʧ��������MnO2�������е���Ԫ����Դ��ˮ������H�����ٽ��缫����ʽ��ƽ���ɡ�
(3)ͼ��X���ĵ缫��ӦΪ2H����2e��=H2��(��2H2O��2e��=H2����2OH��)������X������pH��������������672 mL����֪�õ�����Ϊ0.06 mol��Y����������Ϊ168 mL��ʧ������0.03 mol���ɵ�ʧ�����غ��֪��ʧ������Ϊ0.03 mol���ɵ缫��Ӧ��֪���ܽ�Ϊ0.005 mol����0.28 g��

��ͼ��ʾ��p��qΪֱ����Դ��������A�ɽ�������X�Ƴɣ�B��C��DΪ���缫����ͨ��Դ������X������B����ͬʱC��D�ϲ������ݣ��Իش�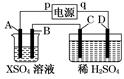
(1)pΪ ����A�������� ��Ӧ��
(2)CΪ �������ռ��� ��DΪ �������ռ��� ��
(3)C���ĵ缫��ӦʽΪ ��
(4)�ڵ������У���C��D�����ϲ�������������ʵ���������±���
| ʱ��(min) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| ������������ �����(cm3) | 6 | 12 | 20 | 29 | 39 | 49 | 59 | 69 | 79 | 89 |
| ������������ �����(cm3) | 2 | 4 | 7 | 11 | 16 | 21 | 26 | 31 | 36 | 41 |
��ϸ��������ʵ�����ݣ���˵���仯�Ŀ���ԭ����
��
(5)����Ӧ����һ��ʱ���A��B�缫������Һ��pH (�������С�����䡱)��
(6)����·��ͨ��0.004 mol����ʱ��B���ϳ����Ľ���XΪ0.128 g����˽�����Ħ������Ϊ ��
�мס�����λͬѧ��������ԭ��ط�Ӧ�������Ļ��˳�����˾���þƬ����Ƭ���缫������ͬѧ���缫����6 mol��L��1��H2SO4��Һ�У���ͬѧ���缫����6 mol��L��1��NaOH��Һ�У���ͼ��ʾ��
��1��д�����������ĵ缫��Ӧʽ_____________________________��
��2�����и���Ϊ________���ܷ�Ӧ�����ӷ���ʽ��
______________________________________________________��
��3�����������ͬѧ����Ϊ������ԭ��صĵ缫����������ǽ������ɸ������ϵĽ���Ӧ�ȹ����������ϵĽ������á�������жϳ�________��Ը�ǿ�����һ��жϳ�________��Ը�ǿ������дԪ�ط��ţ�
��4���ɴ�ʵ��ó������н����У���ȷ����________��
| A������ԭ��ط�Ӧ�жϽ������˳��ʱӦע��ѡ����ʵĽ��� |
| B��þ�Ľ����Բ�һ�������Ľ�����ǿ |
| C����ʵ��˵���������˳����ѹ�ʱ��û��ʵ�ü�ֵ�� |
| D����ʵ��˵����ѧ�о������ӡ���Ӧ������Ӱ��ϴ����Ӧ�������������� |
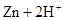 �T�T
�T�T ���ķ�Ӧ���ԭ��ء�
���ķ�Ӧ���ԭ��ء�
 LiNH2+2LiH����������Ϊ ���ѧʽ������270��ʱ���÷�Ӧ���������ų�H2���������﮿���Ϊ������ϣ������������ɴ�Li3N������ %����ȷ��0.1����
LiNH2+2LiH����������Ϊ ���ѧʽ������270��ʱ���÷�Ӧ���������ų�H2���������﮿���Ϊ������ϣ������������ɴ�Li3N������ %����ȷ��0.1����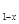 FePO4֮���ת������طŵ�ʱ���������ķ�ӦΪLiXC6��Xe��
FePO4֮���ת������طŵ�ʱ���������ķ�ӦΪLiXC6��Xe�� XLi++6C��д����طŵ�ʱ�ĵ缫��Ӧ�Ļ�ѧ����ʽ ��
XLi++6C��д����طŵ�ʱ�ĵ缫��Ӧ�Ļ�ѧ����ʽ ��