��Ŀ����
��14�֣�ÿ��2�֣�
I����1L������ͨ��CO2��H2��2mol����һ�������·�����Ӧ��CO2 + H2 CO + H2O��
CO + H2O��
�ش��������⣺
��1����830�������£���Ӧ�ﵽƽ��ʱCO2��ת����Ϊ50%�����������ƽ�ⳣ��K1=________��
��2���ڣ�1���Ļ����ϣ�����ϵ�¶Ƚ���800�档��֪�������µ�ƽ�ⳣ��K2=0.81��������֪ �÷�Ӧ������ӦΪ___________��Ӧ������ȡ��������ȡ�����
��3���ڣ�1���Ļ����ϣ�ѹ���������֮0.5 L���������µ�ƽ�ⳣ��ΪK3����K3________K1
L���������µ�ƽ�ⳣ��ΪK3����K3________K1
��4��T��ʱ��ijʱ�̲����ϵ�и����ʵ������£�n��CO2 ��=1.2mol��n��H2��=1.5mol��
��=1.2mol��n��H2��=1.5mol��
n��CO��=0.9mol��n��H2O��=0.9mol�����ʱ�÷�Ӧ ����.
���������Ӧ�������淴Ӧ������ƽ��״̬������
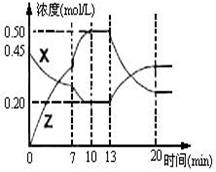
II����һ�ݻ�Ϊ1L ���� �������м���һ������X��Y��������ѧ��ӦX(g)��2Y(s)
�������м���һ������X��Y��������ѧ��ӦX(g)��2Y(s)  2Z(g)����H��0����ͼ��������X��Z�����ʵ���Ũ����ʱ��仯�����ߡ�
2Z(g)����H��0����ͼ��������X��Z�����ʵ���Ũ����ʱ��仯�����ߡ�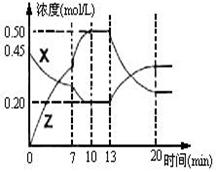
(1)0��10min �����������ѹǿ�� ___________��
����������С������ȷ������
(2)�Ʋ��ڵ�7minʱ���߱仯��ԭ������� ___
��13minʱ���߱仯��ԭ�������  __������ţ�
__������ţ�
������Z���� ������X���� ������
�ܽ��� ��ʹ�ô���
I����1�� �� 1 �� ���� �� ="= "  ������Ӧ����
������Ӧ����
II����1����� ��2�� �ۢ� ��3����
����

 ��У����ϵ�д�
��У����ϵ�д���14�֣�ÿ��2�֣�ij�Լ���������������ͭ�������ᣨ��Fe3+����Ӧ��ȡ���������������£�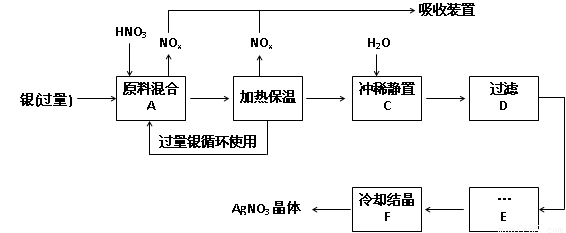
��1����ҵ��һ��ѡ���е�Ũ�ȵ����������Ӧ����ȡ�������������±��ո���ա�
|
|
�ŵ� |
ȱ�� |
|
ʹ��Ũ���� |
��Ӧ���ʿ� |
��Ľϴ���NOx�����϶� |
|
ʹ��ϡ���� |
|
|
��2������B���ȱ��µ������� ��
a�� �����ڼӿ췴Ӧ����
b��������δ��Ӧ������ӷ�
c�������������ַ�Ӧ��������Һ��H+��Ũ��
��3������C��Ϊ�˳�ȥFe3+��Cu2+�����ʣ���ϡʱ����������ԭ���� ��
��4������C�м�ˮ����Ӧ������������������ˮ���Ժ���������ɵIJ���Ӱ���ǣ�
��
��5������E���еIJ����� ��
��6���Ƶõ��������к�����������ͭ��ͨ����ȥ����ͭ�ķ������ڲ���E֮ǰ���������Ƶ�Ag2O��ʹCu2+ת��ΪCu(OH)2��������Ӧ����˳�ȥ���÷�Ӧ�Ļ�ѧ����ʽΪ�� ��
 7N2��12
7N2��12 H2OҲ�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״������ L��
H2OҲ�ɴ���NO2����ת��1.2mol����ʱ�����ĵ�NO2�ڱ�״������ L�� 2SO3��g�� ��H="-196.6" kJ��mol-1
2SO3��g�� ��H="-196.6" kJ��mol-1
 CO + H2O��
CO + H2O�� 2Z(g)����H��0����ͼ��������X��Z�����ʵ���Ũ����ʱ��仯�����ߡ�
2Z(g)����H��0����ͼ��������X��Z�����ʵ���Ũ����ʱ��仯�����ߡ�