��Ŀ����
����Ŀ��ijͬѧ������������ȡ������������Ҫ�ɷ�ΪFe2O3��SiO2��Al2O3�������������������Ʊ���ˮ������������FeSO4��7H2O����������������̣�
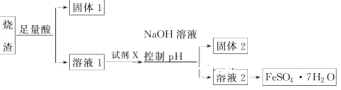
����˵������ȷ����
A���ܽ�����ѡ���������ᣬ�Լ�Xѡ������
B������1��һ����SiO2������pH��Ϊ��ʹAl3��ת��ΪAl��OH��3�������2
C������Һ2�õ�FeSO4��7H2O��Ʒ�Ĺ����У������������ֹ�������ͷֽ�
D�����ı䷽��������Һ1��ֱ�Ӽ�NaOH���������õ��ij����������ܽ⣬����Һ���ᾧ����Ҳ�ɵõ�FeSO4��7H2O
���𰸡�D
��������
���������A�������̷�����֪���ܽ�����ѡ���������ᣬXΪ���ۣ���A��ȷ��B�������̷�����֪������1��һ������SiO2������pHֵʹ��������ȫ�����������������������2Ϊ������������B��ȷ��C�����������ױ������е���������������������ʧȥ�ᾧˮ�����Դ���Һ2�õ�FeSO47H2O��Ʒ�Ĺ����У������������ֹ�������ͷֽ⣬��C��ȷ��D������Һ1�к��������Ӻ������ӣ��ӹ������������ƣ�������ת��Ϊƫ��������ӣ������������������ӽ���������������������������յõ�����������������������������D����ѡD��

 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�����Ŀ��ʵ������Ҫ����0.55mol��L-1NaOH��Һ220mL�������в������������ʵ������֣���ʹ��������������
��1��ѡ����������ɱ�ʵ��������������У�������ƽ��ҩ�ס��ձ�������������
��2�����㡣���Ƹ���Һ��ȡNaOH���塣
��3��������
����ƽ��ƽ��Ӧ����ƽ���������ij��λ�ã�������ͼ����һ�����߱���������Ե������λ�ã�![]() ��1g���������룩��
��1g���������룩��
������������NaOH����Ӧ������ƽ�ģ�������������������������
��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ���� ��
��5��ת�ơ�ϴ�ӡ���ת��ʱӦʹ�ò�����������Ӧע�⣻��Ҫϴ���ձ�2��3����Ϊ�� ��
��6�����ݡ�ҡ�ȡ�
��7������õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�����ñ�ǩ��ע����Һ���ơ�Ũ�ȼ����Ƶ�ʱ�䡣
��8�����в���ʹ������ҺŨ��ƫ����У�����ĸ����ͬ������Ӱ����С�
A������ʱ����������� |
B����NaOH����ֽ�ϳ��� |
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�� |
D��������ƿת��ʱ������Һ�彦�� |
E��δϴ���ܽ�NaOH���ձ�
F������ʱ���ӿ̶���
G������ƿδ���������������Һ