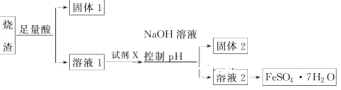
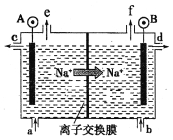
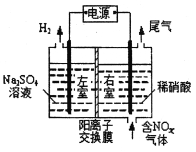
��Ŀ����
����Ŀ��ʵ������Ҫ����0.55mol��L-1NaOH��Һ220mL�������в������������ʵ������֣���ʹ��������������
��1��ѡ����������ɱ�ʵ��������������У�������ƽ��ҩ�ס��ձ�������������
��2�����㡣���Ƹ���Һ��ȡNaOH���塣
��3��������
����ƽ��ƽ��Ӧ����ƽ���������ij��λ�ã�������ͼ����һ�����߱���������Ե������λ�ã�![]() ��1g���������룩��
��1g���������룩��
������������NaOH����Ӧ������ƽ�ģ�������������������������
��4���ܽ⡢��ȴ���ò�ʵ������Ҫʹ�ò�������Ŀ���� ��
��5��ת�ơ�ϴ�ӡ���ת��ʱӦʹ�ò�����������Ӧע�⣻��Ҫϴ���ձ�2��3����Ϊ�� ��
��6�����ݡ�ҡ�ȡ�
��7������õ���Һ����һ��ʱ�����ָ�����Լ�ƿ�����ñ�ǩ��ע����Һ���ơ�Ũ�ȼ����Ƶ�ʱ�䡣
��8�����в���ʹ������ҺŨ��ƫ����У�����ĸ����ͬ������Ӱ����С�
A������ʱ����������� |
B����NaOH����ֽ�ϳ��� |
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�� |
D��������ƿת��ʱ������Һ�彦�� |
E��δϴ���ܽ�NaOH���ձ�
F������ʱ���ӿ̶���
G������ƿδ���������������Һ
���𰸡���1��250ml����ƿ����ͷ�ιܣ�2��5.5��3����![]() ������
������
��4�����裬�����ܽ⣨5��������ĩ�˲��뵽����ƿ�Ŀ̶������£��Ҳ�������������ƿƿ�ڴ����ܽӴ�ƿ�ڣ�������ȫ��ת�Ƶ�����ƿ�У�8��ACG
�������������������1�����������м��㡢�������ܽ⡢��Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ�����һ����������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣨������Ͳ��ȡˮ�����ձ��������ò��������裬�����ܽ⣮��ȴ��ת�Ƶ�250mL����ƿ�У����ò�����������ϴ���ձ���������2-3�Σ�����ϴ��Һ��������ƿ�У���ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�����ݵߵ�ҡ�ȣ���������������������ƽ��ҩ�ס��ձ�����������250mL����ƿ����ͷ�ιܣ������ṩ��������֪������������250ml����ƿ����ͷ�ιܣ�
��2��������220ml������ƿ����Ӧѡ��250ml������ƿ�����Ƴ�250ml����Һ������n=cV��֪��Ҫ��NaOH�����ʵ���n=0.55molL-1��0.25L=0.1375mol������m=nM=0.1375ml��40g/mol=5.5g��
��3����������Ҫ������NaOH��������5.5g��ȷ��ʹ��5g�����������Ŀ̶���0.5g���������λ��Ϊ��![]() ��
��
����ƽ����ʱӦ�������룻
��4���ܽ�����У��������������ǽ��裬�����ܽ⣻
��5���ò���������ʱӦ��������ĩ�˲��뵽����ƿ�Ŀ̶������£��Ҳ�������������ƿƿ�ڴ����ܽӴ�ƿ�ڣ������ϴ���ձ�����ᵼ���ʵ���ʧ����ϴ���ձ���Ŀ���ǽ�����ȫ��ת�Ƶ�����ƿ�У�
��8��A�����������������ʸ���m��=m��+m�����ʳ�������ҩƷ������ƫ��Ũ��ƫ��
B����NaOH����ֽ�ϳ��������׳��⣬����NaOH����ʵ����ƫС��Ũ��ƫС��
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У���ȴ����Һ���ƫС��Ũ��ƫ��
D��������ƿת��ʱ������Һ�彦�����ᵼ�����ʵ���ʧ����Ũ��ƫС��E��δϴ���ܽ�NaOH���ձ����ᵼ�����ʵ���ʧ����Ũ��ƫС��F������ʱ���ӿ̶��ߣ�������Һ���ƫ��Ũ��ƫС��G��������ƿδ���T����������Һ������ҺŨ����Ӱ�죬��ΪֻҪ����ʱ��ȷ������ˮ��ԭ�����еĻ��Ǻ�������ģ���Ũ����Ӱ�졣