��Ŀ����
��15�֣���֪A��B��C��1��18��Ԫ���е�3�֣�0.5molAԪ�ص����ӵõ�6.02��1023�����ӱ���ԭΪ����ԭ�ӣ�0.8gA��������ǡ����100mL0.4mol/L��������ȫ��Ӧ��Aԭ�Ӻ�������������������ȡ�3.6gBԪ�صĵ��ʺ����������ᷴӦ���ų�����4.48L����״����,C�õ�һ�����Ӻ���벵ĵ��Ӳ�ṹ��ͬ��������������ˮ����Ϊ��Ȼ����ǿ���ᡣ
 ��1��д������Ԫ�صķ��ţ�A B______ C______
��1��д������Ԫ�صķ��ţ�A B______ C______
��2����10mLŨ��Ϊ1mol/L��B��C��Ԫ���γɵĻ������ˮ��Һ����ε���35mLŨ��Ϊ1mol/L�Ŀ�������Һ��������___________ ��
���ӷ�Ӧ����ʽΪ___________ �� ��
����������ˡ�ϴ�ӡ��������գ��ɵð�ɫ��������Ϊ ��
 ��1��д������Ԫ�صķ��ţ�A B______ C______
��1��д������Ԫ�صķ��ţ�A B______ C______ ��2����10mLŨ��Ϊ1mol/L��B��C��Ԫ���γɵĻ������ˮ��Һ����ε���35mLŨ��Ϊ1mol/L�Ŀ�������Һ��������___________ ��
���ӷ�Ӧ����ʽΪ___________ �� ��
����������ˡ�ϴ�ӡ��������գ��ɵð�ɫ��������Ϊ ��
. <1>Mg Al Cl ÿ��2��
<2>�����������ܽ⡣ 2��
Al3++3OH- ===Al(OH)3 2��
Al(OH)3 + OH- ==AlO2- + 2H2O 2��
0. 255g 3��
<2>�����������ܽ⡣ 2��
Al3++3OH- ===Al(OH)3 2��
Al(OH)3 + OH- ==AlO2- + 2H2O 2��
0. 255g 3��
��

��ϰ��ϵ�д�
�����Ŀ
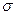 �� h��
�� h��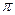 ��
�� 
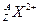 ��������A��137��������N
��������A��137��������N