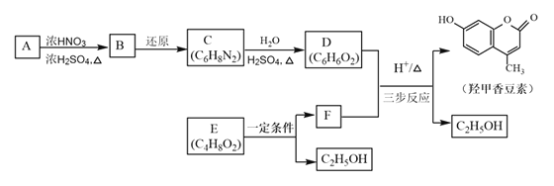
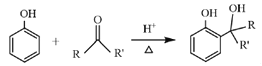
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΡ≥ΙΛ≥ß”Ο»μΟΧΩσ(÷ς“ΣΈΣMnO2Θ§Κ§‘”÷ Al2O3ΚΆ…Ν–ΩΩσ(÷ς“ΣΈΣZnSΘ§Κ§‘”÷ FeS) Ι≤Ά§…ζ≤ζΗ…Βγ≥ΊΒΡ‘≠ΝœZnΚΆMnO2Θ§Ά§ ±ΜώΒΟ“Μ–©Η±≤ζΤΖΘ§ΙΛ“’Νς≥Χ»γœ¬ΘΚ
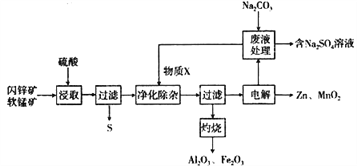
“―÷ΣΘΚΝρΥαΫΰ»Γ“ΚΒΡ÷ς“Σ≥…Ζ÷ΈΣZnSO4ΓΔMnSO4ΓΔFe2(SO4)3ΓΔAl2(SO4)3
Θ®1Θ©ΓΑΫΰ»ΓΓ± ±ΖΔ…ζΒΡΖ¥”Π÷–Θ§ΜΙ‘≠≤ζΈοΈΣ_______________ (ΧνΜ·―ß Ϋ)ΓΘ
Θ®2Θ©ΔΌΓΑΨΜΜ·≥ΐ‘”Γ± ±Θ§Φ”»κΒΡΈο÷ XΩ…ΡήΈΣ______________(Χν―ΓœνΉ÷ΡΗ)ΓΘ
A.MnCO3 B. Zn2(OH)2CO3 C.NaOH D. KOH
ΔΎœύΙΊάκΉ”ΩΣ Φ≥ΝΒμΚΆΆξ»Ϊ≥ΝΒμΒΡpH»γœ¬±μΥυ ΨΘ§Φ”»κXΚσΘ§»ή“ΚpH”ΠΒς’ϊΒΫ________ΓΘ
άκΉ” | ΩΣ Φ≥ΝΒμpH | Άξ»Ϊ≥ΝΒμpH |
Fe3+ | 2.3 | 3.2 |
A13+ | 4.2 | 5.4 |
Zn2+ | 7.2 | 8.0 |
Mn2+ | 8.3 | 9.8 |
Θ®3Θ©÷Μ”Ο“Μ÷÷ ‘ΦΝΘ§Φ¥Ω…¥” ΓΑΉΤ…’Γ±ΥυΒΟΒΡFe2O3ΚΆAl2O3ΜλΚœΈο÷–Ζ÷άκΒΟΒΫFe2O3ΓΘΗΟΖ¥”Π
ΒΡάκΉ”ΖΫ≥Χ ΫΈΣ____________________________________ΓΘ
Θ®4Θ©ΓΑΒγΫβΓ± ±Θ§ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ____________________________________ΓΘ
Θ®5Θ©¥”Na2SO4»ή“Κ÷–ΒΟΒΫΟΔœθΨßΧεΘ®Na2SO4 10H2OΘ©Θ§–η“ΣΫχ––ΒΡ Β―ι≤ΌΉς”–______ΓΔ_____ΓΔΙΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΒ».
Θ®6Θ©ZnΚΆMnO2ΒΡ¥ΪΆ≥…ζ≤ζΙΛ“’÷ς“ΣΨ≠άζΩσ ·±Κ…’ΓΔΝρΥαΫΰ≥ωΓΔΒγΫβΒ»Ιΐ≥ΧΘ§”κ¥ΪΆ≥ΙΛ“’œύ±»Θ§ΗΟΙΛ“’Νς≥ΧΒΡ”≈ΒψΈΣ________________________________ΓΘ
Θ®7Θ©”Ο…ζ≤ζΒΡZnΚΆMnO2÷Τ≥…Η…Βγ≥ΊΘ§‘ΎΦν–‘ΧθΦΰœ¬ΙΛΉς ±”–MnOOH…ζ≥…Θ§‘ρΗΟΒγ≥Ί’ΐΦΪΒΡΒγΦΪΖ¥”Π ΫΈΣ________________________________ΓΘ
ΓΨ¥πΑΗΓΩ MnSO4 AΓΔB 5.4ΓήpH<7.2 Al2O3ΘΪ2OHΘ≠=2AlOΘΪH2O MnSO4ΘΪZnSO4ΘΪ2H2O![]() MnO2ΘΪZnΘΪ2H2SO4 ’τΖΔ≈®Υθ ά以ΫαΨß ΈόSO2Β»Έέ»Ψ–‘ΤχΧε≤ζ…ζΘΜΖœ“ΚΩ…―≠ΜΖάϊ”ΟΘ§‘≠Νœάϊ”Ο¬ ΗΏΘ®ΤδΥϊΚœάμ¥πΑΗ“≤Ω…ΗχΖ÷Θ© MnO2ΘΪH2OΘΪeΘ≠=MnOOHΘΪOHΘ≠
MnO2ΘΪZnΘΪ2H2SO4 ’τΖΔ≈®Υθ ά以ΫαΨß ΈόSO2Β»Έέ»Ψ–‘ΤχΧε≤ζ…ζΘΜΖœ“ΚΩ…―≠ΜΖάϊ”ΟΘ§‘≠Νœάϊ”Ο¬ ΗΏΘ®ΤδΥϊΚœάμ¥πΑΗ“≤Ω…ΗχΖ÷Θ© MnO2ΘΪH2OΘΪeΘ≠=MnOOHΘΪOHΘ≠
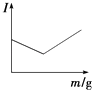
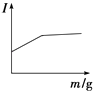
ΓΨΫβΈωΓΩΘ®1Θ©±»ΫœΓΑΫΰ»ΓΓ± ±…Ν–ΩΩσ”κ»μΟΧΩσ÷–‘ΣΥΊΜ·ΚœΦέΒΡ±δΜ·Ω…÷ΣΘ§Mn‘ΣΥΊΜ·ΚœΦέ”…+4ΦέΫΒΒΆΈΣ+2ΦέΘ§Υυ“‘ΓΑΫΰ»ΓΓ± ±ΜΙ‘≠≤ζΈοΈΣMnSO4ΘΜΘ®2Θ©ΔΌΓΑΨΜΜ·≥ΐ‘”Γ± ±Θ§Φ”»κΒΡΈο÷ XΒΡΉς”ΟΨΆ «ΒςΫΎpHΘ§ ΙFe3+ΓΔAl3+≥ΝΒμΆξ»ΪΘ§ΒΪ≤ΜΡή“ΐ»κ–¬ΒΡ‘”÷ Θ§MnCO3ΓΔZn2(OH)2CO3ΡήœϊΚΡ«βάκΉ” ΙpH‘ω¥σΘ§¥ΌΫχFe3+ΓΔAl3+ΒΡΥ°ΫβΘ§ΕχΙΐΝΩMnCO3ΓΔZn2(OH)2CO3“≤≤Μ»ή”ΎΥ°Θ§≤ΔΈΣΚσΟφΒΡ≤ζΤΖΘ§¥πΑΗ―ΓABΘΜΔΎΗυΨί±μ÷––≈œΔΘ§Φ”»κXΚσΘ§ΡΩΒΡ « ΙFe3+ΓΔAl3+ΉΣΜ·ΈΣ≥ΝΒμΘ§ΒΪZn2+ΓΔMn2+≤ΜΡήΩΣ Φ≥ΝΒμΘ§Ι »ή“ΚpH”ΠΒς’ϊΒΫ5.4ΓήpH<7.2ΘΜΘ®3Θ©Al2O3Ρή”κ«ΩΦνΖ¥”ΠΘ§ΕχFe2O3≤ΜΡήΘ§÷Μ”Ο“Μ÷÷ ‘ΦΝΘ§Φ¥Ω…¥” ΓΑΉΤ…’Γ±ΥυΒΟΒΡFe2O3ΚΆAl2O3ΜλΚœΈο÷–Ζ÷άκΒΟΒΫFe2O3ΓΘΗΟΖ¥”ΠΒΡάκΉ”ΖΫ≥Χ ΫΈΣΘΚAl2O3ΘΪ2OHΘ≠=2AlO2-ΘΪH2OΘΜΘ®4Θ©ΗυΨίΆΦ÷––≈œΔΩ…÷ΣΘ§ΒγΫβΚσ≤ζΈο≥ΐ–ΩΚΆΕΰ―θΜ·ΟΧΆβΘ§ΜΙ”–Ρή”κΧΦΥαΡΤΖ¥”Π…ζ≥…ΝρΥαΡΤΒΡΖœ“ΚΘ§ΗΟΖœ“Κ”ΠΗΟ «ΝρΥαΘ§ΕχΖ¥”ΠΈοΈΣΝρΥαΟΧΓΔΝρΥα–Ω»ή“ΚΘ§‘ρΓΑΒγΫβΓ± ±Θ§ΖΔ…ζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚMnSO4ΘΪZnSO4ΘΪ2H2O![]() MnO2ΘΪZnΘΪ2H2SO4ΘΜΘ®5Θ©¥”Na2SO4»ή“Κ÷–ΒΟΒΫΟΔœθΨßΧεΘ®Na2SO4 10H2OΘ©Θ§–η“ΣΫχ––ΒΡ Β―ι≤ΌΉς”–’τΖΔ≈®ΥθΓΔά以ΫαΨßΓΔΙΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΒ»ΘΜΘ®6Θ©¥”¥ΪΆ≥ΒΡΙΛ“’Νς≥Χ…œΩ¥Θ§2ZnS+O2
MnO2ΘΪZnΘΪ2H2SO4ΘΜΘ®5Θ©¥”Na2SO4»ή“Κ÷–ΒΟΒΫΟΔœθΨßΧεΘ®Na2SO4 10H2OΘ©Θ§–η“ΣΫχ––ΒΡ Β―ι≤ΌΉς”–’τΖΔ≈®ΥθΓΔά以ΫαΨßΓΔΙΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΒ»ΘΜΘ®6Θ©¥”¥ΪΆ≥ΒΡΙΛ“’Νς≥Χ…œΩ¥Θ§2ZnS+O2![]() 2ZnO+2SO2ΓΔ2C+O2
2ZnO+2SO2ΓΔ2C+O2![]() 2COΓΔZnO+CO
2COΓΔZnO+CO ![]() ZnΘ®gΘ©+CO2Ζ¥”ΠΕΦ–η“Σ‘ΎΗΏΈ¬œ¬Ϋχ––Θ§“ρ¥ΥΕΦ–η“ΣœϊΚΡ¥σΝΩΒΡΡήΝΩΘΜΆ§ ±”–SO2…ζ≥…ΜαΈέ»ΨΜΖΨ≥ΘΜΕχœ÷‘Ύ–¬ΙΛ“’Ιΐ≥Χ÷–Θ§œϊΚΡΒΡΡήΝΩΫœ–ΓΘ§Εχ«“≤Μ≤ζ…ζ”–ΕΨΒΡΕΰ―θΜ·ΝρΤχΧεΘ§”–άϊ”ΎΜΖ±ΘΘ§Ι ¥πΑΗΈΣΈόSO2Β»Έέ»Ψ–‘ΤχΧε≤ζ…ζΘΜΖœ“ΚΩ…―≠ΜΖάϊ”ΟΘ§‘≠Νœάϊ”Ο¬ ΗΏΘΜΘ®7Θ©”Ο…ζ≤ζΒΡZnΚΆMnO2÷Τ≥…Η…Βγ≥ΊΘ§‘ΎΦν–‘ΧθΦΰœ¬ΙΛΉς ±”–MnOOH…ζ≥…Θ§‘ρΗΟΒγ≥Ί’ΐΦΪMnO2ΒΟΒγΉ”…ζ≥…MnOOHΘ§ΒγΦΪΖ¥”Π ΫΈΣMnO2ΘΪH2OΘΪeΘ≠=MnOOHΘΪOHΘ≠ΓΘ
ZnΘ®gΘ©+CO2Ζ¥”ΠΕΦ–η“Σ‘ΎΗΏΈ¬œ¬Ϋχ––Θ§“ρ¥ΥΕΦ–η“ΣœϊΚΡ¥σΝΩΒΡΡήΝΩΘΜΆ§ ±”–SO2…ζ≥…ΜαΈέ»ΨΜΖΨ≥ΘΜΕχœ÷‘Ύ–¬ΙΛ“’Ιΐ≥Χ÷–Θ§œϊΚΡΒΡΡήΝΩΫœ–ΓΘ§Εχ«“≤Μ≤ζ…ζ”–ΕΨΒΡΕΰ―θΜ·ΝρΤχΧεΘ§”–άϊ”ΎΜΖ±ΘΘ§Ι ¥πΑΗΈΣΈόSO2Β»Έέ»Ψ–‘ΤχΧε≤ζ…ζΘΜΖœ“ΚΩ…―≠ΜΖάϊ”ΟΘ§‘≠Νœάϊ”Ο¬ ΗΏΘΜΘ®7Θ©”Ο…ζ≤ζΒΡZnΚΆMnO2÷Τ≥…Η…Βγ≥ΊΘ§‘ΎΦν–‘ΧθΦΰœ¬ΙΛΉς ±”–MnOOH…ζ≥…Θ§‘ρΗΟΒγ≥Ί’ΐΦΪMnO2ΒΟΒγΉ”…ζ≥…MnOOHΘ§ΒγΦΪΖ¥”Π ΫΈΣMnO2ΘΪH2OΘΪeΘ≠=MnOOHΘΪOHΘ≠ΓΘ

ΓΨΧβΡΩΓΩ”–ΙΊΕΧ÷ήΤΎ‘ΣΥΊAΓΔBΓΔCΓΔDΓΔEΓΔFΒΡ–≈œΔ»γœ¬ΘΚ
‘ΣΥΊ | ”–ΙΊ–≈œΔ |
A | ΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΘ®ΦΉΘ©Ρή”κΤδΤχΧ§«βΜ·ΈοΘ®““Θ©Ζ¥”Π…ζ≥…―Έ |
B | ΉνΆβ≤ψΒγΉ” ΐ «¥ΈΆβ≤ψΒγΉ” ΐΒΡ2±Ε |
C | M≤ψ…œ”–3ΗωΒγΉ” |
D | ΕΧ÷ήΤΎ‘≠Ή”ΑκΨΕΉν¥σΒΡ÷ςΉε‘ΣΥΊ |
E | ΤδΒΞ÷ «Β≠ΜΤ…ΪΙΧΧε |
F | ΉνΗΏ’ΐΦέ”κΉνΒΆΗΚΦέ¥ζ ΐΚΆΈΣ6 |
«κΜΊ¥πœ¬Ν–Έ ΧβΘΚ
Θ®1Θ©–¥≥ω Β―ι “÷Τ»Γ““ΒΡΜ·―ßΖΫ≥Χ Ϋ________________________ΓΘ
Θ®2Θ©œ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «____________(Χν–ρΚ≈)ΓΘ
ΔΌ Β―ι “Ω…”Ο”“ΆΦΥυ ΨΉΑ÷Ο÷Τ»ΓBΒΡΉνΗΏΦέ―θΜ·Έο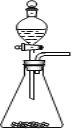
ΔΎ ”ΟCΒΞ÷ Ήω≥…ΒΡ≤έ≥ΒΘ§ΕΦΩ…”Οά¥‘Υ δΦΉΒΡ≈®»ή“Κ
Δέ CΚΆΆ≠”κœΓΝρΥαΉι≥…ΒΡ‘≠Βγ≥ΊΘ§CΒγΦΪ±ΜΜΙ‘≠
Δή DΒΞ÷ ‘Ύ―θΤχ÷–»Φ…’ΚσΒΡ≤ζΈοΩ…”Ο‘ΎΖάΕΨΟφΨΏ÷–ΉςΙ©―θΦΝ
Δί ΙΡάχ≥ΥΉχΙΪΫΜ≥Β≥ω––Θ§≥ΪΒΦΒΆΧΦ…ζΜνΘ§ «ΩΊ÷ΤΚΆ÷ΈάμBO2ΫβΨωΓΑΈ¬ “–ß”ΠΓ±ΒΡ”––ßΆΨΨΕ÷°“Μ
Δό DFΒΡΒγΉ” ΫΈΣ![]()
Θ®3Θ©ΫΪEΒΡ≥ΘΦϊ―θΜ·ΈοΘ®ΗΟ―θΜ·ΈοΡή ΙΤΖΚλ»ή“ΚΆ …ΪΘ©Ά®»κ”…CuSO4ΚΆNaClΜλΚœΒΡ≈®»ή“Κ÷–Θ§»ή“Κ―’…Ϊ±δ«≥Θ§Έω≥ωΑΉ…Ϊ≥ΝΒμΘ§»ΓΗΟ≥ΝΒμΫχ––‘ΣΥΊ÷ ΝΩΖ÷ ΐΖ÷ΈωΘ§Ω…÷ΣΤδ÷–Κ§ClΘΚ35.7%Θ§CuΘΚ64.3%Θ§‘ρΗΟ―θΜ·Έο‘Ύ…œ ωΖ¥”Π÷–ΒΡΉς”Ο «_______ΓΘ
AΘ°Τ·ΑΉΦΝ BΘ°―θΜ·ΦΝ CΘ°ΜΙ‘≠ΦΝ
Θ®4Θ©«κ”ΟΜ·―ßΖΫΖ®Φ”“‘―ι÷ΛΘ®3Θ©÷–ΒΡ―θΜ·ΈοΘ§Φρ“Σ–¥≥ω Β―ιΖΫΖ®ΓΔ ‘ΦΝΦΑ‘ΛΤΎΩ…Ιέ≤λΒΫΒΡœ÷œσ_______ΓΘ