��Ŀ����
[��ѧ��ѡ��ѧ�뼼��]��15�֣�
��ҵ�ϳ��ð��������������ᣬ���������̰������Ĵ�����������Ϊ����Ͻ�˿������һ��������������ˮ���ն��������������ᡣ��ش��������⣺
�Ű���������Ӧ��һ���¶��£�ˮΪ���壩Ϊ����Ӧ���ȵĿ��淴Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ ��ƽ�ⳣ������ʽK= ���������¶ȣ���K��ֵ ���������С�����䡱����
��Ϊ�����NH3��ת���ʣ����Բ�ȡ ��
a����Сѹǿb������Ӧ��Ũ��c��ʹ�ô���d�������¶�e����ʱ�����NH3
��ԭ�����п����������������Ҫԭ���� ��
�Ƚ�����Ͻ����ɱ�˿������Ҫԭ���� ��
��ˮ���ն���������������Ϊ���ȷ�Ӧ���仯ѧ����ʽΪ ��
Ϊ�����ˮ�Զ��������������ʣ��ɲ�ȡ�Ĵ�ʩΪ ����2���
��ҵ�ϳ��ð��������������ᣬ���������̰������Ĵ�����������Ϊ����Ͻ�˿������һ��������������ˮ���ն��������������ᡣ��ش��������⣺
�Ű���������Ӧ��һ���¶��£�ˮΪ���壩Ϊ����Ӧ���ȵĿ��淴Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ ��ƽ�ⳣ������ʽK= ���������¶ȣ���K��ֵ ���������С�����䡱����
��Ϊ�����NH3��ת���ʣ����Բ�ȡ ��
a����Сѹǿb������Ӧ��Ũ��c��ʹ�ô���d�������¶�e����ʱ�����NH3
��ԭ�����п����������������Ҫԭ���� ��
�Ƚ�����Ͻ����ɱ�˿������Ҫԭ���� ��
��ˮ���ն���������������Ϊ���ȷ�Ӧ���仯ѧ����ʽΪ ��
Ϊ�����ˮ�Զ��������������ʣ��ɲ�ȡ�Ĵ�ʩΪ ����2���
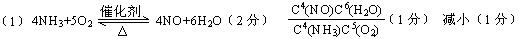 ��2��abd��3����߰���ת���ʺ�һ��������ת���ʣ�4������λ�����Ĵ����뷴Ӧ��ĽӴ������5��3NO2+H2O=2HNO3+NO��ѹ������
��2��abd��3����߰���ת���ʺ�һ��������ת���ʣ�4������λ�����Ĵ����뷴Ӧ��ĽӴ������5��3NO2+H2O=2HNO3+NO��ѹ��������1������д��ѧƽ�ⳣ����ʱ��ע��ˮ��Ҫ©������2�����ݸ÷�Ӧ������Ӧ�����ȷ�Ӧ�����Բ��ý����¶ȵķ������÷�Ӧ������Ӧ���������ķ�Ӧ�����Բ��ü�Сѹǿ�ķ���������Ӧ���Ũ�ȣ���Ӧ����������У���ѡabd����3��������������������������Ũ�ȣ���Ӧ��������NO���ɵķ�����У���4��������״��Ҫ������λ�����Ĵ����뷴Ӧ��ĽӴ��������5������ʽ��3NO2+H2O=2HNO3+NO�����ˮ�Զ��������������ʣ��������NO2��ת���ʣ����Բ��ü�ѹ�����µķ�����

��ϰ��ϵ�д�
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
�����Ŀ
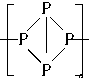 ,�й�����������������ȷ���ǣ�������?
,�й�����������������ȷ���ǣ�������?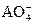 ����Һ�л�ԭ�ɽϵͼ�̬������0.100mol��L-1��H2O2��Һ24.0mL����ͨ������ȷ��AԪ�ص����ռ�̬��
����Һ�л�ԭ�ɽϵͼ�̬������0.100mol��L-1��H2O2��Һ24.0mL����ͨ������ȷ��AԪ�ص����ռ�̬��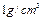 ����ƿ��ˮ��Һ����������ѹΪ
����ƿ��ˮ��Һ����������ѹΪ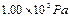 ��������ƿ�в��������ѹǿΪ����Pa������������ѹΪ
��������ƿ�в��������ѹǿΪ����Pa������������ѹΪ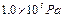 ��
��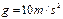 ��
��