��Ŀ����
����Ŀ���������һ�ֳ��õĻ��ʣ��乤ҵ����������ͼ����ش��������⡣

��1��д����Ӧ����B�з�����Ӧ�Ļ�ѧ����ʽ��___��
��2��������C��ͨ�������Ŀ����___���û�ѧ����ʽ���ͣ���C��D������Ӧ�����з����ķ�Ӧ������������ԭ��Ӧ����___ (�Ӧ��������)��
��3��Ũ����һ�㱣������ɫ�Լ�ƿ������������������û�ѧ����ʽ����ԭ��___��
��4��̼��Ũ���ᷴӦ�Ļ�ѧ����ʽ��___��
��5���������������к���NH4+�ķ�����___��
��6����ʢ��12mLNO2��O2�Ļ���������Ͳ������ˮ���У���ַ�Ӧ��ʣ��2mL��ɫ���壬ʣ������������___��___����ԭ���������O2���������Ϊ___mL��___mL��
���𰸡�![]() 4NO+3O2+2H2O=4HNO3 C
4NO+3O2+2H2O=4HNO3 C ![]()
![]() ȡ��Ʒ�������Թܣ�������ˮ�ܽ⣬���Թ��м�������Ũ������������Һ�������Թܣ������Ӽ�סһʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ��������һպ��Ũ����IJ����������Թܿڣ����������̣���˵����Ʒ�к���笠� NO O2 4mL 1.2mL
ȡ��Ʒ�������Թܣ�������ˮ�ܽ⣬���Թ��м�������Ũ������������Һ�������Թܣ������Ӽ�סһʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ��������һպ��Ũ����IJ����������Թܿڣ����������̣���˵����Ʒ�к���笠� NO O2 4mL 1.2mL
��������
��1��B�а���ת��ΪNO�Ļ�ѧ����ʽΪ��![]() ��
��
��2�����������������У�������C��ͨ�������Ŀ���ǽ�һ�����������ɶ���������Ȼ����ˮ��Ӧ����HNO3���䷴Ӧ����ʽΪ��4NO+3O2+2H2O=4HNO3��C��NOת��ΪNO2��������ԭ��Ӧ�����������հ����������ᰱ�Ƿ�������ԭ��Ӧ��
��3��Ũ�������������ֽ⣬����Ӧ���������������ֽ�Ļ�ѧ����ʽΪ��![]() ��
��
��4��C��Ũ�����ڼ��������·�������NO2��CO2��H2O���䷴Ӧ����ʽΪ��![]() ��
��
��5��笠����ӳ������鷽��Ϊ����笠�����ת��Ϊ������Ȼ������ʪ���ɫʯ����ֽ���鰱���������ð�����HCl��Ӧ�����Ȼ�茶��壬����鷽��Ϊ��ȡ��Ʒ�������Թܣ�������ˮ�ܽ⣬���Թ��м�������Ũ������������Һ�������Թܣ������Ӽ�סһʪ��ĺ�ɫʯ����ֽ�����Թܿڣ�����ֽ��������һպ��Ũ����IJ����������Թܿڣ����������̣���˵����Ʒ�к���笠���
��6�����ܷ����ķ�ӦΪ��4NO2+O2+2H2O=4HNO3��3NO2+H2O=2HNO3+NO���Թ���O2��NO2���尴�����1��4���ȫ���ܽ⣬Һ������Թܣ��ٶ�ȫ��Ϊ����������ʣ���������Ϊ![]() ��12mL=4mL��ʵ���ǽ��ʣ��2mL���壬С��4mL����˵��ʣ������ΪNO��������
��12mL=4mL��ʵ���ǽ��ʣ��2mL���壬С��4mL����˵��ʣ������ΪNO��������
��Ϊ��������μӷ�Ӧ������Ϊ12mL-2mL=10mL������4NO2+O2+2H2O=4HNO3����֪�μӴ˷�Ӧ��NO2�����Ϊ10mL��![]() =8mL���μӷ�Ӧ��O2�����Ϊ10mL-8mL=2mL��ԭ���������O2�����Ϊ2mL+2mL=4mL��
=8mL���μӷ�Ӧ��O2�����Ϊ10mL-8mL=2mL��ԭ���������O2�����Ϊ2mL+2mL=4mL��
��ʣ������ΪNO���壬����3NO2+H2O=2HNO3+NO����֪������NO2Ϊ3��2mL=6mL����Ӧ4NO2+O2+2H2O=4HNO3���ĵ����������Ϊ12mL-6mL=6mL����Ӧ���ĵ�����Ϊ6mL��![]() =1.2mL��
=1.2mL��

 �Ķ��쳵ϵ�д�
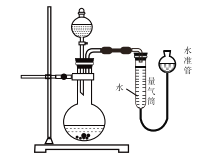
�Ķ��쳵ϵ�д�����Ŀ����֪��KClO3��6HCl(Ũ)=KCl��3Cl2����3H2O����ͼ��ʾ���������Լ��ֱ�����������е���Ӧλ�ã�ʵ��ʱ��Ũ�������KClO3�����ϣ����ñ�����Ǻá��±�����ʵ������ó��Ľ�����ȫ��ȷ����(����)
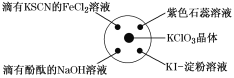
ѡ�� | ʵ������ | ���� |
A | ����KSCN��FeCl2��Һ��� | Cl2���л�ԭ�� |
B | ���з�̪��NaOH��Һ��ɫ | Cl2�������� |
C | ��ɫʯ����Һ�ȱ�����ɫ | Cl2����Ư���� |
D | KI������Һ�����ɫ | Cl2���������� |
A.AB.BC.CD.D