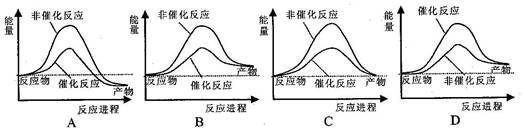
��Ŀ����
��б������������������ͬ�������塣��֪
��S(s����б)��O2(g)=SO2(g) ��H1����297��16 kJ/mol
��S(s������)��O2(g)=SO2(g) ��H2����296��83 kJ/mol
����˵����ȷ���ǣ� ��
| A��������ȵ�б���ȶ� |
| B��S(s����б)=S(s������)��H3����0��33 kJ/mol |
| C����ͬ���ʵ�����������ȵ�б�������е������� |
| D����ʽ��ʾ����1 mol O2�еĹ��ۼ������յ��������γ�1 mol SO2�еĹ��ۼ����ų���������297��16 kJ |
A
�������������A�����ݸ�˹���ɣ���-�ڵõ��Ȼ�ѧ����ʽ��S��s����б��=S��s����������H3=-0��33kJ?mol -1�������Ȼ�ѧ����ʽ��֪���������������ڵ�б�����ʾ��е�����Խ��Խ�ȶ��������������ȶ�����ȷ��B�����ݸ�˹���ɵõ��Ȼ�ѧ����ʽΪ��S��s����б��=S��s����������H3=-0��33kJ?mol -1������C����ͬ���ʵ�����������ȵ�б�������е������ͣ�����D����ʽ��ʾ����lmolO2�й��ۼ��Ͷ���S��s����б�������յ����������γ�1mol SO2�й��ۼ����ų���������297��16KJ������
���㣺�����˸�˹���ɵ�Ӧ�ã������������ȶ��ԵĹ�ϵ�Լ���Ӧ������ܵĹ�ϵ��

����˵����ȷ���ǣ� ��
| A�����ʵĻ�ѧ�仯�������������仯 |
| B���������ȷ�Ӧ����Ҫ���� |
| C���κη�Ӧ�е������仯������Ϊ�����仯 |
| D���ɻ�ѧ�����յ�����һ�������γ��»�ѧ���ų������� |
25�桢101 kPa�£�̼������������������ǵ�ȼ����������393.5 kJ/mol��285.8 kJ/mol��890.3 kJ/mol��2 800 kJ/mol���������Ȼ�ѧ����ʽ��ȷ���� ( )
A��C(s)�� O2(g)=CO(g) ��H����393.5 kJ/mol O2(g)=CO(g) ��H����393.5 kJ/mol |
| B��2H2(g)��O2(g)=2H2O(g) ��H����571.6 kJ/mol |
C�� C6H12O6(s)��3O2(g)=3CO2(g)��3H2O(l)��H����1 400 kJ/mol C6H12O6(s)��3O2(g)=3CO2(g)��3H2O(l)��H����1 400 kJ/mol |
| D��CH4(g)��2O2(g)=CO2(g)��2H2O(g)��H����890.3 kJ/mol |
��֪���ȼ��a g��Ȳ����ʱ����1 mol������̼�����Һ̬ˮ�����ų�����Ϊb kJ������Ȳȼ�յ��Ȼ�ѧ����ʽ��ȷ����( )
| A��2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l)��H=��4b kJ��mol-1 |
| B��C2H2(g)+5/2 O2(g)=2CO2(g)+H2O(l)��H="2b" kJ��mol-1 |
| C��2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l)��H=��2b kJ��mol-1 |
| D��2C2H2(g)+5O2(g)=4CO2(g)+2H2O(l)��H="b" kJ��mol-1 |
����ͼ��ʾ����H1=��393.5 kJ?mol-1����H2=��395.4 kJ?mol-1������˵�����ʾʽ��ȷ����( )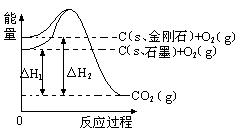
| A��C��s��ʯī��=C��s�����ʯ�� ��H="+1.9" kJ?mol-1 |
| B��ʯī�ͽ��ʯ��ת���������仯 |
| C�����ʯ���ȶ���ǿ��ʯī |
| D��1 molʯī���ܼ��ܱ�1 mol���ʯ���ܼ���С1.9 kJ |
��֪CO��g��+H2O��g�� H2��g��+CO2��g����H��0���������������䣬����˵������ȷ����
H2��g��+CO2��g����H��0���������������䣬����˵������ȷ����
| A������������ı��˷�Ӧ��;������Ӧ�ġ�HҲ��֮�ı� |
| B�������¶ȣ���Ӧ���ʼӿ죬��Ӧ�ų����������� |
| C���ı�ѹǿ��ƽ�ⲻ�����ƶ�����Ӧ�ų����������� |
| D������ԭ����н��У���Ӧ�ų����������� |
����˵����ȷ����( )
A����ͼ�ɱ�ʾˮ�ֽ�����е������仯 |
| B����2C(s)+O2(g)=2CO(g)��H="-221.0" kJ/mol����̼��ȼ����Ϊ110.5 kJ/mol |
| C����Ҫ���ȵķ�Ӧһ�������ȷ�Ӧ���������ܷ����ķ�Ӧһ���Ƿ��ȷ�Ӧ |
| D����֪:��:���ڷ�Ӧ��H2(g)+Cl2(s)="2HCl" (g)��H="-" a kJ/mol�� |

��a��b��c�������㣬��Ͽ�1molH-Cl�����������Ϊ- a-b-c