��Ŀ����
��10�֣��ڳ��³�ѹ�£�����������װ�����ⶨ�������������װ��E���Ե���װ���е���ѹ���Сʵ����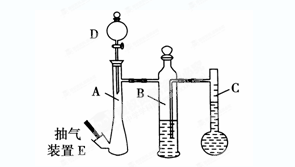
��1������ȡ0.113gþ����10.00 mLϡ����(����)��ȫ��Ӧ��ȡ���������ⶨ1mol H2�����(�ڳ��³�ѹ�²ⶨ)����Ӧ����ʱC�е�Һ��̶�Ϊ128.00 mL��A��δ������ʱC����Һ�壩����ô�������1mol H2�����Ϊ________L(��ȷ��0.001)��
��2���������1mol H2������ȸ��¶Ⱥ�ѹǿ�µ�����ֵƫ�ߵ�ԭ����(���������װ�ö�������)________(ѡ���ţ���ͬ)
A��δ��ȴ�����¡������� B����Ӧ̫��
C��װ�������Բ��� D��Bװ��©��
��3������������װ�òⶨCO2������Ħ�������
��B����ʢ�ŵ�Һ��ӦΪ________��
A������Na2CO3��Һ B������NaHCO3��Һ
C��ˮ D������ʯ��ˮ
������A��D��ʢ�ŵ��Լ�ӦΪ________��
A������ʯ��Ũ���� B����̼���ƣ�ϡ����
C����̼��ƣ�ϡ���� D����̼���ƣ�ϡ����
��4��������װ�ò��������Ħ�������������ֵ������Ϊ��Сʵ�������еĴ�ʩ��_______��
A����Aװ�ý���ʢˮ(����)��ˮ����
B����С���Ũ��
C����Aװ�ý���ʢ��ˮ��ˮ����
D���������Ũ��
��1��n(H2)��n(Mg)��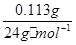 ��V(H2)��128.00 mL��10.00 mL������1mol H2�����Ϊ
��V(H2)��128.00 mL��10.00 mL������1mol H2�����Ϊ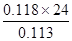 L��
L��
��2���˷�Ӧ�Ƿ��ȷ�Ӧ�������Ӧ���죬��ʹ���ɵ������¶ȸߣ�ˮ���������ߣ����Բ���������ƫ��
��3�������ǻӷ����ᣬ����Ҫѡ�ò��ӷ���ϡ���ᣬ��ϡ���ᷴӦ����̼���ƣ�
��4����С���Ҫ�ӽ��ͷ�ӦҺ���¶����֡�
�𰸣���1��25.062����2��A��B�� ��3����B�� ��B�� ��4��A��B
���������������1��n��H2��=n��Mg��=0.113g/24g?mol?1��V��H2��=128.00 mL-10.00 mL=118.00mL������1mol H2�����Ϊ0.118��24/0.113L=25.062L����2���������ᷴӦ�Ƿ��ȷ�Ӧ�������Ӧ���죬��ʹ���ɵ������¶ȸߣ�ˮ���������ߣ����Բ���������ƫ��ѡAB����3����CO2�뱥��Na2CO3��Һ��ˮ������ʯ��ˮ����Ӧ��Ӱ��ⶨ�������CO2�뱥��NaHCO3��Һ����Ӧ��ѡB���������ǻӷ����ᣬ����Ҫѡ�ò��ӷ���ϡ���ᣬ��ϡ���ᷴӦ����̼���ƣ�ѡB����4��A����Aװ�ý���ʢˮ�����£���ˮ���У��ɽ����¶ȣ���ȷ��B����С���Ũ�ȣ���Ӧʱ�ɽ��ͻ�ѧ��Ӧ���ʣ������¶ȣ���ȷ��C����Aװ�ý���ʢ��ˮ��ˮ���в��ɽ����¶ȣ�����D���������Ũ�ȣ��ܼӿ컯ѧ��Ӧ���ʣ����ɽ����¶ȣ�����ѡAB��
���㣺���������ʵ���Ϊ���ĵļ��㡢��ѧʵ�顣

���������У����ڻ�������
| A��̼���� | B��Һ̬�� | C�����ȼ� | D���������� |
���л������У������ε���
| A��CO | B��MgCl2 | C��H2SO4 | D��KOH |