��Ŀ����
17����֪0.2mol�л����0.4mol O2���ܱ�������ȼ�պ����ΪCO2��CO��H2O��g�������ᆳ��ŨH2SO4����������10.8g����ͨ�����ȵ�CuO����ַ�Ӧ��CuO��������3.2g�����������ͨ����ʯ�ұ���ȫ���գ���������17.6g������֪�����ǻ�����ͬһ��̼�ϲ��ȶ����Զ�ʧȥһ��ˮ����1����ͨ�������ƶϸ��л���ķ���ʽ��
��2����0.2mol���л���ǡǡ����9.2g��������ȫ��Ӧ����ȷ�����л���Ľṹ��ʽ��
��3������Ϊ0.2mol���л���ǡǡ����4.6g��������ȫ��Ӧ����ȷ�����л���Ľṹ��ʽ��
���� ��1��Ũ��������10.8gΪ��Ӧ���ɵ�ˮ��������ͨ����������ͭ�����ڷ�����ӦCuO+CO$\frac{\underline{\;����\;}}{\;}$Cu+CO2����������������3.2g����Ϲ��������仯���ò������ɼ���CO�����ʵ�����ͨ����ʯ��ʱ����ʯ�ҵ�����������17.6g�ɼ�����CO2�����ʵ�������ȥCO��CuO��Ӧ���ɵ�CO2������Ϊ�л���ȼ������CO2������������Ԫ���غ����1mol�л����к���C��H��O�����ʵ�����������û�ѧʽ��
��2����Ϸ���ʽ���л������Ʒ�Ӧ���л������Ƶ����ʵ����Ĺ�ϵ���жϷ����й����ţ��ݴ���д�ṹ��ʽ��
��3��0.2mol���л���ǡǡ����4.6g�����Ƽ�0.2mol�Ʒ�Ӧ����1mol���л���ֻ������1mol�ƣ����л�������к�����1��-OH���ݴ˷�����
��� �⣺��1���л���ȼ������ˮ10.8g�����ʵ���Ϊ$\frac{10.8g}{18g/mol}$=0.6mol��
���л���ȼ�����ɵ�COΪx����
CuO+CO$\frac{\underline{\;����\;}}{\;}$Cu+CO2 ������١�m
28g 16g
x 3.2g
����x=$\frac{28g��3.2g}{16g}$=5.6g��CO�����ʵ���Ϊ$\frac{5.6g}{28g/mol}$=0.2mol��
����̼Ԫ���غ��֪CO��CuO��Ӧ���ɵ�CO2�����ʵ���Ϊ0.2mol������Ϊ0.2mol��44g/mol=8.8g��
�л���ȼ�����ɵ�CO2������Ϊ17.6g-8.8g=8.8g�������ʵ���Ϊ$\frac{8.8g}{44g/mol}$=0.2mol��
����̼Ԫ���غ��֪��1mol�л��ﺬ��̼ԭ�����ʵ���Ϊ$\frac{0.2mol+0.2mol}{0.2}$=2mol��
������Ԫ���غ��֪��1mol�л��ﺬ����ԭ�����ʵ���Ϊ$\frac{0.6mol��2}{0.2}$=6mol
������Ԫ���غ��֪��1mol�л��ﺬ����ԭ�����ʵ���Ϊ$\frac{0.6mol+0.2mol+0.2mol��2-0.4mol��2}{0.2mol}$=2mol��
�����л���ķ���ʽΪC2H6O2��
���л���ķ���ʽΪC2H6O2��
��2����0.2mol���л���ǡǡ����9.2g�����Ƽ�0.4mol��ȫ��Ӧ��˵���л�������к�����2��-OH�����л���Ľṹ��ʽΪHOCH2CH2OH��
�𣺸��л���Ľṹ��ʽΪHOCH2CH2OH��
��3����0.2mol���л�����4.6g�����Ƽ�0.2mol�Ʒ�Ӧ����1mol���л���ֻ������1mol�ƣ����л�������к�����1��-OH������һ��-OHӦ�칹Ϊ�Ѽ����ʴ��л���Ľṹ��ʽΪ��CH3OCH2OH���𣺴��л���Ľṹ��ʽΪCH3OCH2OH��
���� ���⿼���л������ʽ��ȷ�����ѶȲ�������ȼ�շ�����ԭ���غ�ȷ���л���ķ���ʽ�����ݷ�Ӧ��֮�����Ĺ�ϵ�������ṹ�dz�����Ҳ���ѵ㣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�| A�� | ����Ԫ�ص���������ϼ۶����������ڵ������� | |
| B�� | X��Y��X��W�ֱ��γɵļ������Ӷ�ˮ�ĵ����Ӱ������ | |
| C�� | Z2W��Һ�ʼ��Ե�ԭ��ɱ�ʾΪW2-+2H2O?H2W+2OH- | |
| D�� | X��Y��Y��Z�����γɾ���ǿ�����ԵĻ����� |
| A�� |  | B�� | ���� | C�� | ���� | D�� |  |
| A�� | �����£��ܽ�Ũ����ʢ������Ͱ�� | |
| B�� | NH4F��Һ�ܴ���ڲ����Լ�ƿ�� | |
| C�� | ��ȼ����Ҫ�Ǽ�����ˮ�ڵ��¸�ѹ���γɵ�ˮ���ᄃ�壬��˿ɴ����ں��� | |
| D�� | ����պŨ������ް��������Ͱ����Ĺܵ��Ƿ�©�� |
| A�� | ���� | B�� | ��Һ | C�� | ��ȡ | D�� | ���� |
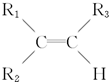 ������Ϊ
������Ϊ ��
��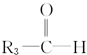 ���ɴ��ƶ�ijϩ������������������CH3CHO��һ����
���ɴ��ƶ�ijϩ������������������CH3CHO��һ����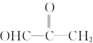 ����ԭϩ���ǣ�������
����ԭϩ���ǣ�������| A�� | CH2�TCHCH2CH��CH3��CH�TCH2 | B�� | CH3CH�TCH-CH�TC��CH3��2 | ||
| C�� | CH3CH�TCHC��CH3���TCHCH3 | D�� | CH3CH�TCHCH�TCHCH��CH3��2 |
| A�� | �ƾ���ˮ | B�� | ���CCl4 | C�� | ˮ�ͱ� | D�� | ���ͺ�ֲ���� |
| A�� | 28g•mol-1 | B�� | 56g•mol-1 | C�� | 84g•mol-1 | D�� | 112g•mol-1 |